Newborn presents with jitters and tremors after delivery
A full-term male infant was born at 40.3 weeks gestational age via vaginal delivery to a 29-year-old single G6 P30204 mother with limited prenatal care (3 visits) and short interval pregnancy. The delivery was precipitous: Rupture of membranes was 3 hours in duration with clear fluid; no intrapartum medications were administered; and the infant’s Apgar scores were 9 and 9 at 1 and 5 minutes, respectively. What's the diagnosis?
Table 1
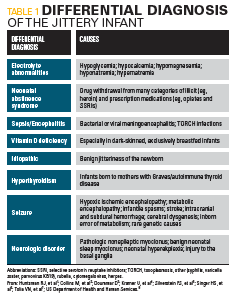
Table 2
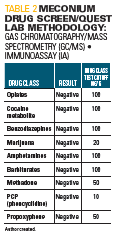
Figure A: Acetaminophen 325 mg/oxycodone hydrochloride
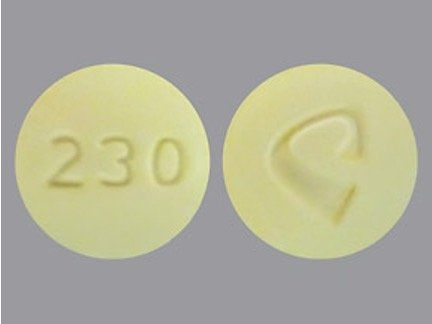
Figure B: Percocet

Title 3
The case
A full-term male infant was born at 40.3 weeks gestational age via vaginal delivery to a 29-year-old single G6 P30204 mother with limited prenatal care (3 visits) and short interval pregnancy. The delivery was precipitous: Rupture of membranes was 3 hours in duration with clear fluid; no intrapartum medications were administered; and the infant’s Apgar scores were 9 and 9 at 1 and 5 minutes, respectively.
In the delivery room, the infant was noted to be very jittery with oscillatory tremor of both upper and lower extremities. Upon further questioning, the mother said that she had been prescribed Percocet by her dentist, which she had used approximately twice daily for the past 3 weeks for dental pain. She also endorsed tobacco smoking (4 to 5 cigarettes per day) but denied alcohol or other prescription or illicit drug use. Her intrapartum urine drug screening was negative.
A full prenatal lab profile for the infant was not available at the time of delivery, but prenatal labs drawn were subsequently found to be: AB positive blood type with negative antibody screen; HIV negative; RPR nonreactive; HBsAg negative; MRSA culture negative; sickle test negative; and rubella immune. Gonorrhea/chlamydia and group B Streptococcus (GBS) cultures were not done.
The infant’s weight was 3240 g, gestational age examination was consistent with full term status, and he plotted appropriately for all other growth parameters on the growth chart (weight, 45%; head circumference, 50%; length, 50%).
The infant’s initial point-of-care glucose testing was normal (at 83 mg/dL) and he was left with the mother for skin-to-skin time and breastfeeding. Six hours later on routine rounds, the infant‘s examination revealed significant hypertonia, irritability, and jitteriness (high-frequency rhythmic tremors involving all extremities that were able to be stopped with firm but gentle pressure of the extremity). His mother also stated he was not latching well on the breast and had ingested only 10 mL of supplemental formula.
Differential diagnosis
The differential diagnosis for a newborn with jitteriness is shown in Table 1.
The most common electrolyte abnormality seen in infants with jitteriness is hypoglycemia. Point-of-care glucose testing on this infant remained normal, and he did not have any of the other obvious associated risk factors for hypoglycemia (such as being large for gestational age [LGA] or small for gestational age [SGA]). Other electrolyte testing was within normal limits.1
Neonatal abstinence syndrome typically is seen in opiate withdrawal, but it encompasses withdrawal from a wide range of both illicit (heroin, cocaine, amphetamines, phencyclidine [PCP], ethyl alcohol [EtOH]) and prescription drugs (including prescription opiates, benzodiazepines, barbiturates, and selective serotonin reuptake inhibitors [SSRI] antidepressants).
Jitteriness and seizures both can be signs of neonatal sepsis but usually in conjunction with respiratory distress, heart rate, and temperature instability. Risk factors for sepsis include premature rupture of membranes (PROM) occurring up to 18 hours before birth; maternal intra-amniotic infection often manifesting as maternal fever during labor; colonization with GBS; and preterm delivery.
Vitamin D deficiency recently has been highlighted as a cause for persistent jitteriness or chin tremor later than 1 week of age in darker-skinned, exclusively breastfed infants. Many dark-skinned mothers especially in northern climates have low vitamin D levels themselves despite taking prenatal vitamin supplementation.2
Benign jitteriness of the newborn can occur in healthy neonates during the first 2 weeks of life. It is usually a rhythmic tremor of high frequency and low amplitude, involving the chin and extremities, stimulus sensitive and exacerbated by crying, with normal neurologic findings and associated with normal development and neurologic outcome.3
Hyperthyroidism in the neonate can be present in the context of maternal Graves disease or other autoimmune hyperthyroidism and should be suspected in infants presenting with tachycardia, jitteriness, diarrhea, and possibly goiter.
Neonatal seizures can be subtle and need to be differentiated from jitteriness, although the etiology of both can be similar. Seizures should be suspected if there are associated autonomic changes such as apnea, abnormal ocular phenomena, or lipsmacking movements, and inability to be suppressed by restraining the affected body part. The underlying causes of seizures are numerous, but suspicion is aroused in infants with hypoxic ischemic encephalopathy (manifested by low Apgar scores at delivery) and with abnormal inborn error of metabolism screens. (This infant’s newborn metabolic screen was normal.) Infants with suspicion for seizures would need electroencephalogram (EEG) monitoring, combined with cranial imaging, usually magnetic resonance imaging (MRI) and anticonvulsants.4,5
In infants with persistent jitteriness without obvious acute etiology, underlying rarer neurologic causes should be sought in conjunction with a pediatric neurologist.6
Hospital course
The infant was subsequently transferred to the Transitional Nursery for further monitoring of his significant neurologic signs and symptoms. Comprehensive metabolic panel testing showed normal electrolyte values (Na, 138; K, 5.5; Ca, 10.6; BUN, 8; Cr, 0.36; glucose, 57; chloride, 106; CO2, 22; anion gap, 16; protein, 5.6; albumin, 3.8; ALP, 168; ALT, 14; AST, 33). Further preprandial point-of-care glucose testing was initiated and remained normal (>70 mg/dL) and partial sepsis screening was negative.
The differential diagnosis of a jittery infant is extensive. Based on the history and clinical findings, infant urine and meconium drug testing were sent with a working diagnosis of neonatal abstinence syndrome (NAS).
The NAS scoring using the modified Finnegan scoring system placed the infant’s first 24-hour scores at a range of 16 to 21 due to:
1. Central nervous system (CNS) symptoms: continuous high-pitched cry, sleeping less than 1 hour between feeds, very overactive Moro reflex, tremors undisturbed, increased muscle tone;
2. Metabolic/respiratory/vasomotor symptoms: temperature greater than 99.4°F, frequent yawning and sneezing; and
3. Gastrointestinal (GI) disturbances: excessive sucking and poor feeding.
Oral morphine was started at a dose of 0.1mg/kg/dose every 4 hours. By the second day of life, the patient’s scores reduced to 12 to 14 with clinical improvement by 48 hours of age to scores less than 8. The infant was fed with 22 kcal/oz formula in anticipation of his increased caloric demands.
Other appropriate nonpharmacologic interventions for NAS were concurrently instituted such as recruiting a “volunteer cuddler” when available to provide comfort and kangaroo care. The infant also received physical therapy for the marked hypertonia, which improved significantly over the hospital course.
Additional lab results/history
The infant’s urine and meconium drug screens were both negative for all categories tested. Table 2 lists the meconium drug screen results.
The medical chart review with the Obstetrics team could not validate the mother’s report of any dental prescription for Percocet. Upon further questioning, the mother’s story became more inconsistent regarding her drug use. It changed from being “prescribed Percocet by a dentist” to her purchasing Percocet “on the street for pain relief,” and once again to her taking “a small round, yellow pill provided by her friend who recently had extra pills from a cesarean delivery” a month prior.
The mother further elucidated that she took these yellow pills for pain in conjunction with naproxen, extra-strength Tylenol, and Robitussin. Searching the drugs.com image data base (www.drugs.com/imprints.php) for “round yellow pill” that was in the category of pain reliever revealed “C 230 (acetaminophen 325 mg and oxycodone hydrochloride 10 mg)” supplied by Alvogen, and a yellow oval pill “Percocet 10/325” supplied by Endo Pharmaceuticals (Figure). Both pills had imprints on each side; however, the mother stated she did not recall any imprints on the pills she took. Despite frequent attempts to have the mother provide a sample of the pill or a picture of her friend’s pill bottle, she remained noncompliant to clinicians’ requests.
Discussion
Neonatal abstinence syndrome is one of the fastest-growing diagnoses in newborn medicine7 and is a result of the sudden discontinuation of fetal exposure to substances that were used or abused by the mother during pregnancy. According to a national survey conducted in the United States in 2012, 5.9% of pregnant women use illicit drugs, 8.5% drink alcohol, and 15.9% smoke cigarettes.8 The most commonly used substance in pregnancy is nicotine, followed by alcohol, marijuana, and cocaine with a recent five-fold increase in opiate use in pregnancy, coincident with prescription opiate misuse.9
The American College of Obstetrics and Gynecology (ACOG) recommends universal drug testing for pregnant women.10 If universal drug testing is not undertaken, guidelines for medically indicated newborn drug testing include:
1. Infants whose mothers have any of the following: (a) history of drug abuse in present or previous pregnancies; (b) limited prenatal care (<5 prenatal visits); (c) history of hepatitis B, AIDS, syphilis, gonorrhea, prostitution; (d) unexplained placental abruption; (e) unexplained premature labor.
2. Infants who have any of the following: (a) unexplained neurologic complications (eg, intracranial hemorrhage or infarction, seizures); (b) evidence of possible drug withdrawal (eg, hypertonia, irritability, seizures, tremulousness, muscle rigidity, decreased or increased stooling); (c) unexplained intrauterine growth retardation.11
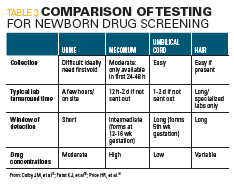
Testing a neonate who presents with NAS can be conducted using various tissue samples (Table 3).
Urine testing sensitivity is low because of problems encountered in urine collections and the high drug thresholds used in current urine assays. Meconium, which begins to form between the 12th and 16th week of gestation, can be used in drug testing; however, it detects maternal drug use only during the last 4 to 5 months of pregnancy. Although unlikely, drugs administered during labor and delivery may be detected in meconium.
Additional sampling can involve umbilical cord tissue, which has the advantage of being available immediately after birth, and the collection process is simple. The disadvantage is that the mechanism of drug deposition into cord tissue is not well understood, and studies have found that drugs and metabolites do not accumulate in cord tissue to the same extent as in meconium.12
Hair sampling can be done at birth because hair grows during the third trimester. Therefore, detection of drugs in newborn hair reflects maternal drug use during the last 3 months of pregnancy.13 Because hair analysis is technically demanding and confounded by drug deposit in hair, hair type, environmental contamination, and limited amount of neonatal hair, the availability of newborn hair testing is limited to a few specialized laboratories.14
With reference to this case, synthetic opioids (fentanyl and methadone) are not detected by immunoassay urine drug testing. Semisynthetic opioids (oxycodone, oxymorphone, buprenorphine, and hydromorphone) may not be well detected or may be inconsistently detected by screening immunoassay urine drug screen. Both synthetic and semisynthetic opioids are detected by confirmatory gas chromatography/mass spectrometry. An expanded opiate panel is needed to detect other commonly used narcotics, including fentanyl (Duragesic), hydrocodone (Hycodan), methadone, oxycodone (Roxicodone, OxyContin), buprenorphine, and tramadol (Ultram).15
The interpretation of neonatal toxicology results can be challenging for physicians who must integrate multiple patient results (mother and newborn) and multiple specimen types (urine, meconium, umbilical cord, hair) while evaluating prenatal prescription medication lists, intrapartum medications, and mother’s self-reported history. In this case, the clinical presentation of the infant, in combination with the mother’s later claim that she self-medicated with Percocet for up to 1 month prior to delivery, led the clinicians to treat the infant with morphine for presumed NAS secondary to opiate withdrawal. Confounding the diagnosis was the clinicians’ inability to detect opiates in both the mother’s and infant’s drug screens.
Clinicians need to know the limitations of drug screening in their practice. Firstly, the type of assay used is important, as immunoassay urine drug screen often is not able to detect synthetic and semisynthetic opiates well. Secondly, in this case, inconsistencies in the mother’s history made it hard to know exactly what she was taking, and testing for “designer drugs” (eg, synthetic cathinones and cannabinoids) is challenging due to continual changes in synthetic compounds and increasing numbers of novel substances.15 These substances are being used with little known about their effects in pregnancy and on the neonate, and significant difficulty in detecting them with routine laboratory tests. Third, urine drug specimens can be tampered with to produce a false-negative result, and although this infant’s specimens were collected in the nursery and therefore were not subjected to abuse, that may not be the case for collection of maternal samples. Lastly, detection of drug use depends on the pattern and frequency of drug(s) used by the mother. When she last used and duration of detectability all play a role. It was likely she had stopped using a few days prior to delivery as the infant was essentially exhibiting withdrawal symptoms almost immediately after birth, so it was not unexpected that the drug could already have been cleared from both the mother’s and infant’s systems.
Summary
In this case, the infant was weaned off morphine based on his Finnegan scoring and discharged home to his mother after Child Protective Services’ evaluation was completed. The infant was scheduled for follow-up with his pediatrician, and a recommendation was provided for referral to early intervention services and possibly to a developmental pediatrician (based on the infant’s attainment of milestones) as an outpatient.
Jitteriness is a common condition in the newborn that typically presents after the first few hours of life. In this case, jitteriness was noted immediately in the delivery room to an infant born to a mother whose risk factor was recent “prescription” opioid use. As his symptomatology worsened, the history became increasingly inconsistent, making it difficult to elucidate exactly what drug was used by the mother and whether it had been prescribed. Confounding the knowledge of what drug was implicated in clinical signs of NAS were the accompanying negative drug screens on both the mother and neonate.
There are many variables that impact how, why, and when an infant will experience neonatal abstinence symptoms. These include the timing and frequency of the mother’s recent intake of a drug, maternal and placental metabolism, and the presence of poly-substance use including cigarettes, methadone, SSRIs, and benzodiazepines. For the infant, withdrawal symptoms will vary depending on gender, gestational age, and genetic factors that influence the infant’s metabolism and excretion of the drug. Additionally, drug testing is becoming increasingly complex with newer modalities in use that require the general pediatrician to be aware of both test- and laboratory-specific variations and limitations.
With multiple synthetic and semisynthetic prescription opioids, as well as an expanding category of so-called “designer” recreational synthetic drugs that are flooding the market, there can be an overwhelming clinical presentation of NAS despite lack of laboratory evidence. In such cases the pediatrician should remain on high alert. A referral still needs to be made to social work and Child Protective Services especially if the results of the toxicology screens are unexpected or incongruent with the overall clinical picture.
Acknowledgements: The author thanks Dr. Inez Reeves and Dr. Michal Young for their review and editing of the manuscript.
References:
1. Huntsman RJ, Lowry NJ, Sankaran K. Nonepileptic motor phenomena in the neonate. Paediatr Child Health. 2008;13(8):680-684.
2. Collins M, Young M. Benign neonatal shudders, shivers, jitteriness, or tremors: early signs of Vitamin D deficiency. Pediatrics. 2017;140(2:e20160719.
3. Doummar D. Benign jitteriness of newborn. Medecine Therapeutique Pediatrie. 2011;14:82-83.
4. Kramer U, Nevo Y, Harel S. Jittery babies: a short-term follow-up. Brain Dev. 1994;16(2):112-114.
5. Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62(2):112-120.
6. Singer HS, Mink JW, Gilbert DL, Jankovic J. Movement disorders in childhood. 2nd ed. Academic Press; 2016.
7. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126.
8. US Department of Health and Human Services; Substance Abuse and Mental Health Services Administration; Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health, 2012 (ICPSR 34933). Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], Published November 23, 2015. Accessed April 15, 2020. https://doi.org/10.3886/ICPSR34933.v3
9. Hayes MJ, Brown MS. Epidemic of prescription opiate abuse and neonatal abstinence. JAMA. 2012;307(18):1974-1975.
10. American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice. Committee opinion: Opioid use and opioid use disorder in pregnancy. No. 711. Published August 2017. Accessed April 15, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/opioid-use-and-opioid-use-disorder-in-pregnancy
11. ARUP Laboratories. Newborn drug testing-meconium and umbilical cord tissue. ARUP Consult website. Updated March 2020. Accessed April 15, 2020. https://arupconsult.com/content/newborn-drug-testing
12. Colby JM, Cotton SW. Facing challenges in neonatal drug testing: How laboratory stewardship enhances care for a vulnerable population. Clinical Laboratory News website. Published March 1, 2018. Accessed April 15 2020. https://www.aacc.org/publications/cln/articles/2018/march/facing-challenges-in-neonatal-drug-testing
13. Farst KJ, Valentine JL, Hall RW. Drug testing for newborn exposure to illicit substances in pregnancy: pitfalls and pearls. Int J Pediatr. 2011;2011:951616.
14. Price HR, Collier AC, Wright TE. Screening pregnant women and their neonates for illicit drug use: consideration of the integrated technical, medical, ethical, legal, and social issues. Front Pharmacol. 2018;9:961.
15. Moeller KE, Kissack JC, Atayee RS, Lee KC. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
11-year-old boy with testicular pain and rash
January 19th 2024An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. Read the full case to see if you can correctly diagnose the patient.
Newborn with midline neck lesion
December 21st 2023A 4-day-old boy with a midline neck lesion was born at term by normal vaginal delivery. After birth, mid line lesion had the configuration of a linear cleft with a cephalocaudal orientation, extending from the level below the hyoid bone to the suprasternal notch with a length of 2.5 cm and a width of 0.5 cm. What's the diagnosis?
A 13-year-old girl with well-demarcated rash on back and chest
October 19th 2023A healthy 13-year-old girl presented with a 1-month history of an asymptomatic, well-demarcated rash on her back and upper chest. The eruption consisted of discrete, dark brown papules that coalesced into large, flat-topped plaques with mild superficial scale and accentuation of skin markings. What's the diagnosis?
Suspicious facial swelling in a 22-month-old girl
October 11th 2023A 22-month-old female patient with sickle cell disease on folic acid and penicillin prophylaxis with a 3-day history of nasal congestion, rhinorrhea, fever and decreased oral intake presents to the emergency department (ED) for acute facial swelling noted when she woke up from a nap. What's the diagnosis?
Friction-induced blistering on a child’s feet
July 14th 2023You are called to the hospital nursery to evaluate a healthy full-term newborn boy who developed painful flaccid blisters and erosions on the tops of his feet and ankles shortly after birth. His mother had a history of similar recurrent skin lesions that healed with scarring. She also had oral and gastrointestinal tract involvement. What's the diagnosis?