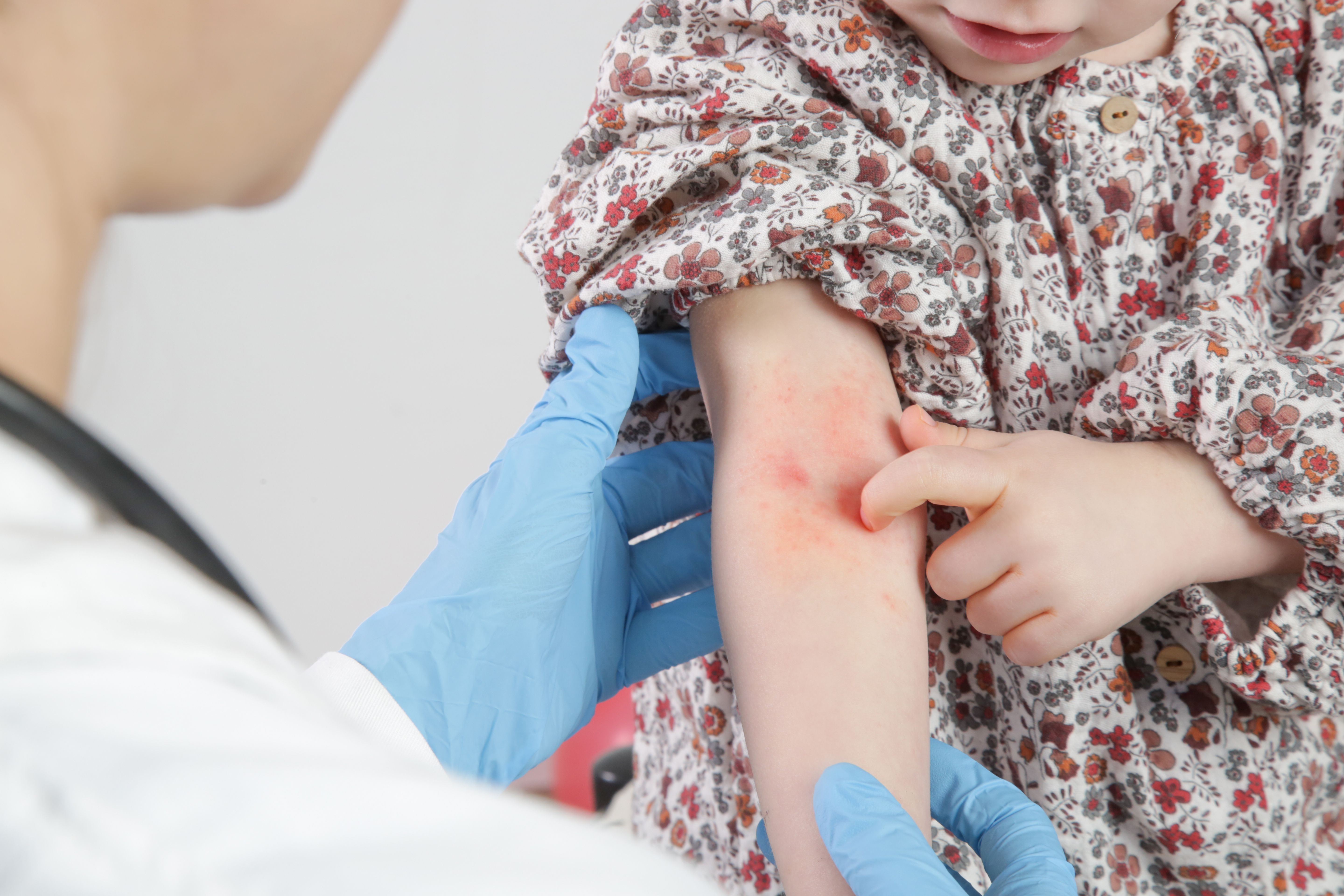
Tapinarof cream safe, effective for pediatric atopic dermatitis
Results from a recent phase 3 clinical trial demonstrated topline safety and efficacy results for tapinarof cream, 1% (VTAMA; Dermavant) as a potential treatment for atopic dermatitis in patients 2 years and older.
Tapinarof cream, 1% (VTAMA; Dermavant) showed positive topline results in the phase 3 ADORING 2 (NCT05014568) study as a potential treatment for children as young as 2 years with moderate to severe atopic dermatitis (AD).
Tapinarof is a novel, aryl hydrocarbon receptor agonist developed as a once-daily, nonsteroidal topical cream to treat AD. In the study, pediatric and adult patients with AD were randomized in a 2:1 ratio to receive thertapinarof cream or vehicle cream once daily.
In ADORING 2, 46.4% of participants treated with tapinarof achieved the primary endpoint of a Validated Investigator Global Assessment scale for Atopic Dermatitis (vIGA-AD) score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline at week 8 (P < 0.0001).
Additionally, 59.1% of patients treated with tapinarof achieved the key secondary endpoint of a proportion of subjects with greater than or equal to 75% improvement in the Eczema Area and Severity Index (EASI) (P < 0.0001) at week 8. Patients 12 years or older with a baseline Peak Pruritus Numerical Rating Scale (PP-NRS) score of 4 or greater, achieved 4-point or greater reduction in PP-NRS at week 8.
“Atopic dermatitis can have a negative impact on the quality of life of diagnosed children as well as their families,” said Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, California. “Both the efficacy and itch data from the ADORING 2 trial are highly encouraging in this regard, pointing to [tapinarof] cream as a potential nonsteroidal topical treatment option for AD that is safe and well tolerated in children. Importantly, the potential to use the same dose regimen with [tapinarof] cream for children and adults with AD offers treatment simplicity for prescribers, helped even more by the fact that it is the same regimen already being used for plaque psoriasis.”
In the study, tapinarof cream demonstrated no safety or tolerability signals. Adverse events were mild to moderate, with a low study discontinuation rate because of adverse events (1.5% tapinarof vs 3% vehicle). Contact dermatitis (1.1% tapinarof vs 1.5% vehicle) and follicular event (8.9% tapinarof vs 1.5% vehicle) were adverse events of special interest.
Currently, tapinarof is approved for the topical treatment of plaque psoriasis in adults. Both adults and pediatric patients receiving tapinarof in the study did so at the same dose and dose regimen as the already approved cream for adults.
Recently, Dermavant released results from a pediatric maximal usage pharmacokinetics (MUPK) study of tapinarof cream in AD. In that study, minimal to no systemic exposure was demonstrated despite maximal use.
“The topline results from ADORING 2 underscore [tapinarof] cream as a potential well-tolerated therapeutic with a favorable safety profile,” said Linda Stein Gold, MD, director of Clinical Research and the Division Head of Dermatology at the Henry Ford Health System, Detroit, Michigan. “When one considers this phase 3 data alongside the recently reported pediatric maximal usage pharmacokinetic (MUPK) AD study, which in treated patients demonstrated minimal-to-no-systemic exposure despite heavy disease burden, [tapinarof] cream is positioning itself to be a potential 2-in-1 first-line topical treatment for both atopic dermatitis and plaque psoriasis.”
Reference
Dermavant reports positive topline results from ADORING 2 atopic dermatitis phase 3 trial of VTAMA (tapinarof) cream, 1% once daily in adults and children as young as 2 years old. Dermavant. March 15, 2023. Accessed March 17, 2023. https://www.businesswire.com/news/home/20230315005395/en/Dermavant-Reports-Positive-Topline-Results-from-ADORING-2-Atopic-Dermatitis-Phase-3-Trial-of-VTAMA%C2%AE-tapinarof-Cream-1-Once-Daily-in-Adults-and-Children-as-Young-as-2-Years-Old
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.