Update on the 2007 National Asthma Education and Prevention Program: Guidelines for the Treatment of Asthma in Children
The National Heart, Lung, and Blood Institute and National Asthma Education and Prevention Program (NAEPP) released its Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma--Full Report, in August 2007.1 The EPR-3 is the fourth iteration of the guidelines, which were first released in 1991 (EPR-1), revised in 1997 (EPR-2), and partially revised in 2002 (Update on Selected Topics). For the first time since their inception, the guidelines include separate recommendations specific to children aged 0 to 4 years and 5 to 11 years. Table 1 highlights the key differences between the 1997 EPR-2 guidelines and the 2007 EPR-3 guidelines regarding treatment of pediatric asthma.

Table 1
The National Heart, Lung, and Blood Institute and National Asthma Education and Prevention Program (NAEPP) released its Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma--Full Report, in August 2007.1 The EPR-3 is the fourth iteration of the guidelines, which were first released in 1991 (EPR-1), revised in 1997 (EPR-2), and partially revised in 2002 (Update on Selected Topics). For the first time since their inception, the guidelines include separate recommendations specific to children aged 0 to 4 years and 5 to 11 years. Table 1 highlights the key differences between the 1997 EPR-2 guidelines and the 2007 EPR-3 guidelines regarding treatment of pediatric asthma.
This review provides the clinician with an overview of treatment and monitoring recommendations for children in both age groups.
INTRODUCTION
The new guidelines emphasize that asthma is a variable disease, with symptoms that change over time in any one patient and with differences among patients and age groups.1,2 Sources of asthma variability include physiological, environmental, and behavioral factors (Table 2).3 The EPR-3 states that "the course of asthma may vary markedly between young children, older children and adolescents, and adults, and this variation is probably more dependent on age than on symptoms."1 Indeed, an analysis of 5 double-blind, randomized, 12-week trials in children aged 4 to 11 years (N = 276) who were previously treated with short-acting b2-adrenergic agonists (SABAs) and subsequently randomly assigned to the placebo maintenance arm for any of these studies, demonstrated that these children frequently change severity categories: in more than 35%, severity changed more than 15 times.4 This study provides evidence of the limitations of classifying asthma based solely on severity, which can change over time.

Table 2
In contrast with earlier iterations of the guidelines, the EPR-3 recommends that physicians base treatment selection and adjustments on the level of asthma control and responsiveness to therapy, rather than just severity.1 Classification of asthma severity is emphasized for initiation of therapy in patients not currently receiving controller medications, whereas assessing control is emphasized for monitoring and adjusting therapy. Moreover, the EPR-3 changed the class of "mild intermittent" asthma to "intermittent" asthma to stress that even patients with intermittent disease can have severe exacerbations.
As in the 1997 and 2002 guidelines, asthma severity should still be classified based on asthma symptoms, reliever use of SABAs, exacerbations, and pulmonary function. A key difference between the 2007 and earlier reports, however, is that asthma severity and control are now defined in terms of 2 domains: impairment and risk.1 The impairment domain includes the present effects of asthma on traditional functional capacity indices (eg, symptoms) and quality of life. Risk is based on the likelihood of future adverse outcomes. Each domain may respond differently to treatment.
DIAGNOSIS OF ASTHMA IN CHILDREN
The 2007 EPR-3 report includes recent evidence showing that asthma begins early in life with a recognizable pattern of risk factors.1 The report acknowledges that asthma in preschool-aged children is often underdiagnosed and undertreated because the symptoms of asthma are similar to those of bronchitis, pneumonia, and upper respiratory tract infections. On the other hand, the majority of children who wheeze or have symptoms of asthma before 3 years of age do not experience symptoms after 6 years of age.1,5 To help distinguish between children whose symptoms will remit in the preschool years and those who will have persistent asthma throughout childhood, the guidelines recommend use of the modified asthma predictive index (API).1,6-9 Accordingly, persistent disease is likely to be present in children younger than 3 years who have had 4 or more wheezing episodes in the previous year and who have either 1 of the following major risk factors: a parental history of asthma, physician-diagnosed atopic dermatitis, or evidence of sensitization to aeroallergens, or 2 of the following minor risk factors: evidence of sensitization to foods, 4% or greater peripheral blood eosinophilia, or wheezing apart from colds.

Table 3
To establish a diagnosis of asthma, physicians also should determine whether episodic symptoms of airflow obstruction or airway hyperresponsiveness are present, establish that airflow obstruction is at least partially reversible, and exclude alternative diagnoses.1 A thorough medical history, physical examination, and pulmonary function testing (in children 5 years or older) are recommended. Spirometry is preferred to peak flow measurements in the diagnosis of asthma primarily because of the variability in predicted peak flow reference values.1 However, as many pediatricians' offices are not equipped for spirometry testing, the physician may need to arrange testing through a local pulmonary function laboratory. It has been demonstrated that spirometry testing can be performed successfully in the office setting by pediatricians when they have access to the appropriate spirometry equipment and training.10 During the medical history-taking process, the clinician can identify symptoms that suggest asthma and determine other factors that support this diagnosis, such as a family history of asthma or allergies.
Table 3 provides some sample questions for the diagnosis and initial assessment of asthma. Physical examination findings should focus on those that increase the probability of an asthma diagnosis, such as hyperexpansion of the thorax.
In children aged 5 years or older, the EPR-3 recommends that the spirometry measurements of forced expiratory volume in 1 second (FEV1), FEV in 6 seconds, forced vital capacity (FVC), and FEV1/FVC be measured before and after the patient inhales a SABA to demonstrate pulmonary obstruction and assess reversibility.1
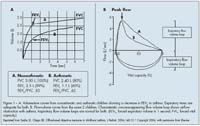
provides examples of spirometric curves for patients with asthma.10 An increase in FEV1 of 12% or greater from baseline or an increase of 10% or greater of predicted FEV1 after administration of a SABA indicates reversibility.1 For children aged 0 to 4 years, who typically cannot perform spirometry or peak flow, a therapeutic trial of controller medications aids in the diagnosis of asthma (see Pharmacological Treatment Recommendations, Persistent Asthma: Children Aged 0 to 4 Years, Initiation of Long-term Control Therapy).1
DETERMINANTS AND ASSESSMENT OF ASTHMA CONTROL
Once the diagnosis of asthma has been established, the EPR-3 recommends periodic reassessment of asthma control. The EPR-3 places a stronger emphasis on asthma control than previous guideline versions.1 The goal of asthma therapy is to achieve long-term control by reducing impairment and risk. Within the impairment domain, this goal includes preventing chronic and troublesome symptoms, decreasing the need for symptomatic use of SABAs to no more than 2 days a week; maintaining near normal pulmonary function; achieving normal levels of exercise, play, and day-care or school attendance; and meeting patients' and families' satisfaction with treatment. Within the risk domain, the goal is to prevent recurrent exacerbations to minimize the need for emergency care or hospitalization, reduce the risk of reduced lung growth in children (measured by a prolonged failure to attain age-appropriate predicted lung function values), and minimize the adverse effects of pharmacotherapy.
The EPR-3 recommends ongoing monitoring of the signs and symptoms of asthma; pulmonary function (spirometry and peak expiratory flow [PEF]); quality of life/functional status; history of asthma exacerbations; pharmacotherapy for adherence and potential adverse effects; and patient-provider communication and patient satisfaction.1 In general, spirometry testing should be performed periodically (ie, at least every 1 to 2 years) to assess airway function and to check the quality of PEF measurements.1 However, more frequent spirometry testing may be necessary in patients who demonstrate poor response to treatment. Daily PEF monitoring is useful in the assessment of treatment response and in the early detection of changes that may indicate asthma worsening, particularly in patients who have poor perception of asthma control or a history of severe exacerbations.1 Monitoring of asthma control with minimally invasive markers, such as exhaled nitric oxide and sputum eosinophils, requires further evaluation before they can be recommended as clinical tools for routine asthma management.1,3
Asthma control can be assessed by the clinician and through patient self-assessment.1 In patients whose asthma control is being maintained, follow-up is recommended at 1- to 6-month intervals. The EPR-3 guidelines recommend that patients with intermittent asthma or mild or moderate persistent asthma that has been controlled for 3 months or longer be seen by a clinician every 6 months, while those with uncontrolled or severe persistent asthma be seen more frequently. However, the frequency of scheduled visits to monitor asthma control is a matter of clinical judgment.
The EPR-3 also recommends that clinicians encourage patients to use self-assessment tools to determine whether asthma is controlled from the perspective of the patient and/or the patient's family. Patient assessment tools include daily diary cards and self-assessment forms.1,3 These tools are easily accessible to patients and their families (adolescents 12 years and older: http://www.sleepworkplay. com/; children aged 4 to 11: http://www.asthmacontrol.com/ AsthmaControlTestChild.html).12,13
PHARMACOLOGICAL TREATMENT RECOMMENDATIONSGeneral Principles

The EPR-3 guidelines provide a stepwise approach to therapy in which the type, amount, and dosage of medications are increased and decreased as needed to maintain long-term asthma control with the least amount of medication.1 These stepwise recommendations and suggestions for therapy are based on categories of evidence (Table 4). Compared with the previous version of the guidelines, the number of steps has been expanded from 5 to 6, and components within each step have been simplified. Stepwise recommendations for children aged 0 to 4 and 5 to 11 years (Figure 2) are newly separated from those for adolescents 12 years and older and adults.
An additional change from EPR-2 to EPR-3 is that the determination of the appropriate step of care for a child depends on whether therapy is being initiated for the first time or whether therapy is being adjusted.1 Initiation of long-term control therapy is based on classifying asthma severity before treatment (Figure 3) using the impairment and risk domains and selecting the corresponding step of treatment. Inhaled corticosteroids (ICSs) are the preferred therapy when initiating long-term control therapy in children of all ages (Evidence A).
Treatment decisions after therapy has been initiated or for children already receiving medication should be based on the patient's response to therapy and the level of asthma control achieved in the impairment and risk domains (Evidence B; Figure 4).1 Children aged 0 to 4 years are often symptom-free between exacerbations, thus the risk domain is a stronger indicator of morbidity than the impairment domain. The level of impairment is generally based on the most severe symptom. In addition to symptoms, assessment of impairment also includes asthma control scores from a validated instrument (if available) and pulmonary function test results for children aged 5 to 11 years. In these older children, if spirometry suggests poorer control than other measures, fixed airway obstruction should be considered; if that is not the cause, a step-up in therapy should be considered because low FEV1 is a predictor of exacerbation.
In all children, therapy should be stepped up if necessary to achieve control and stepped down to the minimum medication necessary to maintain control.1 For all children at all steps of care, a SABA should be taken as needed to relieve symptoms (Evidence A). Use of a SABA more than 2 days per week for symptom control (not including exercise-induced bronchospasm) or increasing use indicates the need for long-term control therapy in those not receiving such, or a need to step up controller therapy. Before stepping up therapy, patient adherence and technique in using their medications should be assessed and addressed (Evidence C).
Intermittent Asthma
The first step of care corresponds with intermittent asthma for both pediatric age groups. Treatment with as-needed SABAs is usually sufficient for step 1 care1; however, some children with intermittent disease may need additional treatment. For children aged 0 to 4 years experiencing exacerbations from viral respiratory infections, SABA treatment scheduledevery 4 to 6 hours for 24 hours, or longer with physician consult, is recommended for mild exacerbations, with consideration of a 3- to 10-day course of oral systemic corticosteroids (OSCs) (1 mg/kg/d of prednisone or equivalent) for moderate to severe exacerbations. For children aged 0 to 4 and 5 to 11 years who have a history of severe exacerbations with viral respiratory infections, an OSC may be considered at the first sign of infection (Evidence D). In addition, a detailed written action plan should be developed for patients with intermittent asthma and a history of severe exacerbations (Evidence B).
Some patients may have an asthma phenotype that is not truly intermittent. The Acute Intervention Management Strategies (AIMS) trial characterized children aged 12 to 59 months (N = 238) who within the previous 12 months had recurrent (2 or more) episodes of wheezing associated with a respiratory tract infection and either 2 wheezing episodes resulting in an urgent care visit, 2 wheezing episodes requiring OSC, or 1 wheezing episode of each type.14 The results of the AIMS study showed that 71% of children experienced 4 or more wheezing episodes and 60% received at least 1 course of OSCs in the previous year, despite having minimal or no symptoms in the previous month. The AIMS study results indicated that the majority of the children had intermittent asthma interspersed with episodes of acute severe wheezing, suggesting a distinct asthma phenotype.14 Although the EPR-3 guidelines do not specifically address management of this phenotype, they do recommend periodic monitoring to evaluate whether a child truly has intermittent disease.1 In the opinion of the EPR-3, children who experience 2 or more exacerbations per year that require OSC treatment with no or minimal symptoms in the interim are considered to have intermittent impairment but a persistent risk of exacerbation. The EPR-3 suggests that these children be treated as having persistent disease, even in the absence of an impairment level consistent with persistent asthma (Evidence D).
Persistent Asthma: Children Aged 0 to 4 Years
Initiation of long-term control therapy.The EPR-3 recommends that daily controller therapy be considered for use only during periods of previously documented risk for a child (Evidence D); however, if daily long-term control therapy is discontinued after a season of increased risk, the patient should be scheduled for an appointment 2 to 6 weeks after therapy discontinuation to determine whether adequate control is being maintained.1 In addition, a written action plan that indicates the signs of worsening asthma and the appropriate actions to take should be reviewed with the caregivers (Evidence D). Long-term control therapy should be considered for infants and young children who have a second asthma exacerbation requiring systemic corticosteroids within 6 months (risk) or who require symptomatic treatment more than 2 days per week for more than 4 weeks (impairment) (Evidence D).
In addition, long-term control therapy is recommended for infants and young children who have had 4 or more episodes of wheezing in the past year that lasted longer than 1 day and affected sleep and who have risk factors for persistent asthma based on the modified API (Evidence A).1 This recommendation is based on the results of a 36-month randomized, double- blind, placebo-controlled trial in children (N = 285) aged 2 to 3 years considered to be at high risk for developing asthma based on the modified API.7 During the 24-month treatment period, children who received fluticasone 88 µg twice daily via a pressurized metered-dose inhaler (pMDI) experienced significantly more episode- free days (P = .006) and fewer exacerbations requiring treatment with systemic corticosteroids (P < .001) than did children who received placebo.7 However, the proportion of episode-free days and the number of exacerbations regressed to baseline after discontinuation of study drug, with no significant differences between groups after the 12-month observation period. Thus, the use of an ICS does not change disease progression; however, the NAEPP notes that it is important to administer ICS therapy early in the course of the disease to reduce disease impairment and risk.1
A low-dose ICS is the preferred daily long-term control therapy for infants and young children who have never been treated with long-term therapy.1 Because recommendations are based on limited data, however, treatment is often in the form of a therapeutic trial. Therefore, close monitoring of the child's response to therapy is recommended. If no clear beneficial response occurs within 4 to 6 weeks and medication technique and adherence are satisfactory, treatment should be discontinued and an alternative therapy or diagnosis considered (Evidence D).
If a clear and positive response exists for 3 months or more, therapy should be stepped down to the lowest possible doses of medication required to maintain asthma control (Evidence D). In addition, the need for ICS therapy should be reevaluated given the high rates of spontaneous remission of symptoms in this age group. A step down to intermittent therapy as needed for symptoms may be considered (Evidence D). As with use of therapy during periods of risk, written action plans should be reviewed and follow-up appointments scheduled.
Stepwise treatment recommendations. For persistent asthma, the EPR-3 recommends daily long-term control medications that have anti-inflammatory effects (Evidence A).1 Recommendations for preferred therapy with supporting categories of evidence are summarized in Table 5. Low-dose daily ICS therapy is preferred for step 2 care (Figure 2), based on studies of individual drug efficacy in this age group (Evidence A). Long-term clinical studies in children younger than 3 years have demonstrated that ICS therapy is more effective than placebo (n = 285)7 or cromolyn (n = 625).1,15
Alternative step 2 treatments, which are listed alphabetically, include cromolyn (Evidence B, extrapolated from studies in older children) and montelukast (Evidence A).1 Although cromolyn treatment was shown to reduce the risk of asthma-related hospitalization in patients aged 0 to 17 years in a retrospective cohort study,16 it is rarely prescribed by pediatricians. A study in children aged 2 to 6 years (N = 335) demonstrated that nebulized budesonide inhalation suspension was significantly more effective than nebulized cromolyn in reduc- ing asthma exacerbations, improving symptoms, and decreasing rescue medication use (all P < .001).17 Moreover, a systematic review that included some studies in children younger than 5 years demonstrated that cromolyn treatment provided inconsistent symptom control.18
In children aged 2 to 5 years, daily treatment with montelukast (4 or 5 mg/d based on age) has improved measures of asthma control in patients with persistent disease19 and reduced exacerbation rates and the rate of short-term episodic-use ICS courses, but not OSC bursts in children with a history of intermittent asthma symptoms.20 Montelukast may be considered in children 2 years and older when inhaled medication delivery is suboptimal because of poor technique or adherence.1 If an alternative therapy is used and asthma control is not achieved and maintained over 4 to 6 weeks, the preferred ICS treatment should be tried before stepping up therapy.
For step 3 care, the EPR-3 recommends increasing the ICS dose to the medium range to ensure that the adequate dose is delivered before adding adjunctive therapy (Evidence D).1 For example, a trial of children aged 12 to 47 months (N = 237) with moderate asthma showed a trend toward dose-dependant improvements in many diary-related variables for fluticasone propionate 200 µg/d and 100 µg/d administered with a pMDI plus a valved holding chamber compared with placebo, but differences between the fluticasone doses were not significant.21 In that study, the percentages of children with at least 1 exacerbation were 37%, 26%, and 20% for placebo, fluticasone 100 µg/d, and 200 µg/d, respectively; differences between fluticasone 200 µg/d and placebo, as well as the dose- related order effect, were significant. In the 3 pivotal trials for nebulized budesonide inhalation suspension (BIS) that included children 6 months to 8 years of age (N = 1018), dosages of 0.25 mg once daily to 1.0 mg twice daily were more effective than placebo in improving symptoms and reducing rescue medication use, but a dose-response trend was only observed in one of the trials.22
In summary, few studies in this age group have evaluated the effectiveness of increasing the ICS dose, and the findings have been mixed.21-23
The EPR-3 notes that data on adding a long-acting b2-adrenergic agonist (LABA) (salmeterol or formoterol) at step 3 of care are limited for this age group.1 In children aged 4 to 16 years whose asthma was not well controlled on ICS therapy alone, Russell and coworkers24 demonstrated that the addition of salmeterol dry powder inhaler (DPI) to ICS therapy improved pulmonary function and symptom control compared with placebo. However, the number of children aged 4 years was small and precludes any accurate extrapolation from these findings to children aged 0 to 4 years.1
For step 4 care, a medium-dose ICS and adjunctive therapy with either montelukast or a LABA are preferred based on extrapolation of data from studies in older children and adults (Evidence D), because no data were found addressing this issue in children aged 0 to 4 years.1 A recent placebo-controlled study (not available during the NAEPP's review of the evidence) in which 94% of children were receiving an ICS with or without a LABA demonstrated that the addition of montelukast to usual asthma therapy reduced symptomatic days and unscheduled physician visits during the September asthma exacerbation season in children aged 2 to 14 years.25 The effect was greatest among 2- to 5-year-old boys.
A high-dose ICS and either montelukast or a LABA are recommended for step 5, and a high-dose ICS and either montelukast or a LABA and an OSC may be given for step 6. These recommendations are based on expert opinion (Evidence D) because data for step care in young children are lacking.1 At step 6, a 2-week course of OSCs should be considered to confirm clinical reversibility and responsiveness to therapy before these agents are given long-term; a high-dose ICS in combination with both a leukotriene receptor antagonist (LTRA) and a LABA may be considered for children 4 years old. For patients who require long-term OSC use, physicians should prescribe the lowest dose possible, monitor patients closely for adverse events, persistently attempt to reduce the OSC dose after symptoms are controlled, and recommend consultation with an asthma specialist.
Persistent Asthma: Children Aged 5 to 11 Years
Stepwise recommendations for therapy in children aged 5 to 11 years (Figure 2) are generally similar to those for children aged 0 to 4 years, with long-term control therapy recommended for those with persistent asthma (Evidence A).1 In addition to use in children with persistent symptoms, initiation of ICS therapy also may be considered for those who experience frequent and severe exercise-induced bronchospasm.
The main difference between age groups in the recommendations is in steps 3 and 4 (see Table 5). Also, at step 5, the addition of either montelukast or LABA to ICS therapy is the preferred treatment option for children aged 0 to 4 years, whereas add-on LABA therapy is preferred for those aged 5 to 11 years. Another difference is the recommendation for subcutaneous allergen immunotherapy (SCIT) at steps 2 through 4 for children aged 5 to 11 years whose asthma symptoms are known to be related to specific allergens.1 These differences are generally based on the availability of controller therapy studies in older children. Additional studies in older children also are available to support recommendations that are similar for both pediatric age groups, such as step 2 care.
For step 2 care in children aged 5 to 11 years, the preferred treatment is daily low-dose ICS therapy (Evidence A), with cromolyn, an LTRA, nedocromil, and theophylline as alternative treatments (Evidence B).1 Three studies in children aged 6 to 14 years with persistent asthma demonstrated that ICS therapy was more effective than montelukast in a variety of asthma control measures, including pulmonary function, asthma symptoms, and rescue medication use.26-28 In another study, Szefler and associates29 demonstrated that children aged 6 to 17 years who had lower pulmonary function or higher levels of markers of allergic airway inflammation were more likely to respond positively to ICS therapy than montelukast therapy, whereas children without these characteristics may respond similarly to both medications.
Data comparing ICS treatment with theophylline are limited; however, Reed et al30 reported better asthma outcomes with an ICS compared with theophylline in a randomized trial of 185 children with asthma. If alternative treatment is required, montelukast requires once-daily dosing, whereas cromolyn and nedocromil require administration 4 times a day and have shown inconsistent efficacy. The LTRA zafirlukast has several potential drug interactions and a small risk of hepatoxicity.31 Theophylline is rarely prescribed by pediatricians because of the potential for adverse effects associated with toxicity and the need to adjust dosages based on diet, drug interactions, and age. However, the EPR-3 indicates that this agent may be considered when cost and adherence to inhaled medications are of concern, but only if serum levels are monitored closely.1
For step 3 care in this age group, physicians have 2 equally weighted preferred treatment options that are based on the extrapolation of studies in adults (Evidence B): either a medium-dose ICS or a low-dose ICS plus adjunctive therapy (ie, LABA, LTRA, or with appropriate monitoring, theophylline).1 Increasing the ICS dose to the medium-dose range is supported by a systematic review in children 4 to 16 years of age (N = 1733) that demonstrated additional efficacy of fluticasone propionate 400 µg/d (medium dose) in children with severe asthma, despite a dose-response plateau between 100 and 200 µg/d (ie, low dose) for improvements in pulmonary function and symptom control.32 In addition, a trial of budesonide DPI 100, 200, or 400 µg administered twice daily in children aged 6 to 18 years with moderate to severe asthma showed a small dose-response effect on FEV1, morning PEF, and daytime asthma symptoms, but not nighttime asthma symptoms.33 These studies did not assess whether children whose asthma was not controlled on low-dose ICS therapy experienced improved outcomes after increasing the dose. In an adult study, however, treatment with budesonide DPI 800 µg/d significantly reduced exacerbations (risk) and decreased impairment (less rescue medication use, improved symptoms) compared with budesonide 200 µg/d.34
One of the preferred options listed in step 3 is the addition of a LABA to ICS therapy. This combination was shown to improve pulmonary function and symptom control compared with placebo in 2 studies in children whose asthma was not completely controlled with ICSs.24,35 The study by Russell and associates24 that included children aged 4 to 16 years is discussed in the treatment section for 0- to 4-year-old children.
In children aged 6 to 11 years, Zimmerman and colleagues35 demonstrated that addition of formoterol DPI 4.5 or 9 µg twice daily to ICS therapy improved pulmonary function compared with placebo. Furthermore, a meta-analyses of 8 randomized controlled trials in patients aged 5 to 17 years failed to show a significant reduction in asthma exacerbations with the addition of a LABA to maintenance ICS therapy (percentage receiving ICS varied by study) plus placebo or a SABA.36 Although a study in children aged 6 to 16 years with moderate asthma showed no added benefit of adding salmeterol 50 µg twice daily to therapy with beclomethasone 200 µg twice daily, these children may not have been candidates for step-up therapy.37 The FDA approval of ICS/LABA in children aged 4 to 11 years is mainly based on pediatric safety data38,39 and extrapolation of efficacy studies from adults and adolescents.
Addition of an LTRA or theophylline to ICS therapy also is discussed in step 3. The addition of montelukast 5 mg to the ICS budesonide (400 µg/d) in children aged 6 to 14 years (N = 279) significantly reduced as-needed SABA use and improved most measures of pulmonary function.40 In children 6 to 18 years of age (N = 36), a small improvement in PEF was observed with the addition of theophylline to ICS therapy compared with placebo; however, there were no differences in symptoms or rescue medication use.41 Theophylline is considered the less desirable adjunctive treatment option because of the risk of toxicity, multiple drug interactions, and the need for regular monitoring of serum concentrations.1
For step 4 care, medium-dose ICS and LABA treatment is preferred (Evidence B, extrapolated from studies in children aged 12 years or older and adults). These studies in adolescents and adults show that adding a LABA to ICS therapy improves pulmonary function, and many children aged 5 to 11 years whose asthma is not well controlled on step 3 treatment may have low pulmonary function.1 Recommendations for alternative treatment, addition of either an LTRA or theophylline to medium-dose ICS therapy, also are extrapolated from studies in children aged 12 years or older and adults (Evidence B). Although no studies in children younger than 11 years compare the effects of an add-on LABA versus an add-on LTRA, comparative studies in adolescents and adults (Evidence A) support LABAs as preferred. If a physician chooses to add an LTRA for a therapeutic trial because of concerns with LABA treatment, and that trial shows a lack of effectiveness, theophylline could be added with attention to the therapeutic caveats noted previously. The EPR-3 notes that if the selected adjunctive therapy does not lead to improvement in asthma control, its use should be discontinued and a trial of a different add-on therapy should be assessed before therapy is stepped up to the next level.
At steps 2 through 4, SCIT is recommended for patients with allergic asthma.1 A meta-analysis of data from 75 randomized clinical trials demonstrated that SCIT significantly reduced asthma symptoms and that 4 patients would need to be treated to avoid one deterioration in asthma symptoms.42 Furthermore, Mller and colleagues43 showed that in children aged 6 to 14 years who had seasonal rhinoconjuctivitis without asthma at baseline (n = 151), a 3-year course of allergen-specific immunotherapy reduced the risk of asthma development. A recent 5-year follow-up from that trial showed that these benefits persisted: those children treated with immunother- apy had significantly fewer asthma symptoms 2 years after therapy discontinuation.44
The preferred (LABA) and alternative (LTRA or theophylline) treatments for step 5 care are based on extrapolation of studies in older children and adults (Evidence B), whereas the step 6 care recommendations are based on expert opinion (Evidence D).1 The same therapeutic considerations for step 6 OSC therapy that apply to children aged 0 to 4 years also apply to those aged 5 to 11 years (eg, 2-week trial first, monitoring). In addition, pulmonary function should be measured to assess OSC response; alternative or concomitant pulmonary conditions should be considered if the response is poor.
DIFFICULT-TO-TREAT ASTHMA
The new EPR-3 guidelines do not provide treatment recommendations for children with difficult-to-treat asthma who may be at steps 5 and 6 of care. The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) observational study of patients with difficult-to-treat or severe asthma in children aged 6 to 17 years showed that these patients have high rates of health care use and loss of pulmonary function despite using multiple controller medications.43 These findings suggest that additional strategies and intervention programs are needed for these children.
The EPR-3 recommends referral to an asthma specialist for consultation or co-management (Evidence D) if there are difficulties achieving or maintaining control of asthma.1 In addition, an asthma specialist should be consulted for children aged 0 to 4 years who require step 3 care or higher and those aged 5 to 11 years who require step 4 care or higher. Finally, referral also should be considered for patients who require hospitalization for an exacerbation, when immunotherapy or other immunomodulators are considered, or when additional tests are indicated to determine the role of allergy.
Medications
Table 6 provides EPR-3 dosage recommendations for long-term control medications for children aged 0 to 11 years.1Table 7provides the estimated comparative daily doses for ICSs in children. Of note, the EPR-3 dosage recommendations are based on the EPR-3's review of the published evidence, not solely on the age recommendations and dosages approved by the FDA. Doses, particularly those in the high range, may be outside of approved labeling. The EPR-3 highlights 5 long-term control medications that are approved for young children: 2 ICSs, nebulized BIS for children aged 1 to 8 years and fluticasone DPI for children 4 years and older; the LTRA montelukast (based on safety rather than efficacy data) 4-mg chewable tablets for children aged 2 to 6 years and 4-mg granules for those as young as 1 year; the ICS/LABA salmeterol/fluticasone DPI combination for children 4 years and older; and cromolyn nebulizer solution for those 2 years and older.
SAFETY OF ICSs IN CHILDREN
The EPR-3 guidelines state that ICSs are the most effective long-term therapy available for mild, moderate, or severe persistent asthma and are generally safe and well tolerated at the recommended dosages (Evidence A).1 The potential risks of adverse events from ICS treatment are well balanced with their benefits. Local adverse effects of ICSs include oral candidiasis, dysphonia, reflex cough, and bronchospasm. To reduce the potential for these local adverse effects, the EPR-3 recommends the use of spacers or valved holding chambers used with non-breath-activated MDIs (Evidence A); however, no data on the use of spacers with ultrafine particle hydrofluoroalkane (HFA) MDIs were available for the EPR-3 evidence-based review. To reduce the risk of oral candidiasis, patients should be advised to rinse their mouths after inhalation (Evidence B).
A reduction in growth velocity may occur in children as a result of poorly controlled asthma or from the use of corticosteroids. Although studies have shown that low- to medium-dose ICSs may decrease growth velocity, these effects are small (approximately 1 cm in the first year of treatment), generally not progressive, and may be reversible (Evidence A).1,7,46,47 Early intervention studies with fluticasone propionate or budesonide showed significantly improved asthma outcomes despite a small reduction in growth velocity.7,48 Notably, the only long-term prospective growth studies were with budesonide--the EPR-3 has generalized these findings to other ICSs.1 The EPR-3 recommends that children receiving ICS therapy should be monitored for changes in growth (Evidence D).
In children, low- to medium-dose ICSs do not have clinically significant effects on hypothalamic-pituitary-adrenal axis function, glucose metabolism, or the incidence of subcapsular cataracts or glaucoma.1 Moreover, low and medium doses of ICSs have not been shown to have serious adverse effects on bone mineral density (BMD) in children.1,46,47,49 However, data in adults are conflicting50-52 and suggest a cumulative effect of ICSs on BMD.53 BMD may be measured every 1 to 2 years based on duration and dose of ICSs, OSC use, and BMD score (Evidence D). Age-appropriate dietary intake of calcium and exercise should be reviewed with the child's caregivers (Evidence D).1
Although ICSs are generally safe when administered at their recommended dosages, physicians need to recognize the potential for spacers to increase the systemic availability of ICSs and the potential for adverse events with high ICS dosages.1 A recent study showed that use of an antistatic valved holding chamber with face mask increased lung bioavailability of fluticasone delivered from an HFA MDI compared with use of a conventional valved holding chamber and face mask.54 Twelve children aged 1 to 6 years previously maintained on fluticasone chlorofluorocarbon MDI 220 µg twice daily, defined by the NAEPP as a high daily dose, received the same dosage regimen via HFA MDI from 2 types of spacers in a crossover study. Mean steady-state fluticasone plasma concentrations were 107 ± 30 pg/mL and 186 ± 134 pg/mL for the conventional and antistatic holding chambers, respectively. Large interpatient variability in dosage increases occurred, with use of the antistatic holding chamber increasing fluticasone plasma concentration by 100% or more in 5 patients.
In a questionnaire study in the United Kingdom, 27 of 28 children who experienced acute adrenal crisis had been treated with high-dose fluticasone propionate, even though fluticasone was the least prescribed ICS in the United Kingdom. In these children, the mean daily dose of fluticasone was 980 µg, which is considered a high daily dose by the NAEPP (more than 352 µg/d).1,55
Overall, the efficacy of ICSs outweighs concerns about growth or other systemic effects. Nonetheless, the EPR-3 recommends that ICSs should be titrated to the lowest dose of ICS needed to maintain control of a child's asthma.1 Before increasing the dose of ICS, the EPR-3 recommends evaluation of patients' adherence and inhaler technique as well as environmental factors that may contribute to asthma severity (Evidence B). The EPR-3 also recommends consideration of adding a LABA to a low- or medium-dose ICS rather than increasing the ICS dose to achieve or maintain control (Evidence A, ages 5 to 11 years; Evidence D, ages 0 to 4 years).
PARTNERSHIP FOR ASTHMA CARE
A partnership between the patient and clinician is recommended to promote effective asthma management (Evidence A).1 To form a network of support and ensure that all patients are equipped with the knowledge and skills needed to adequately control their disease, patients with asthma should be educated at multiple points of care where they interact with health professionals (Evidence A or B depending on point of care). Family members, health care professionals, and individuals who come into regular contact with a patient with asthma (eg, teachers, coaches, day-care workers, employers) also should receive asthma education to help reduce asthma morbidity and mortality and to promote earlier diagnosis of the disease.
Patient education is an essential part of successful asthma management. Caregivers and children should be educated about asthma, what defines well-controlled asthma, the role of medication, device use, environmental control measures, and what to do when they have signs and symptoms of worsening asthma.1 The EPR-3 recommends that asthma self-management education be included for children with asthma (Evidence A). Sample written action plans are available in the EPR-3 document(http://www.nhlbi.nih.gov/ guidelines/asthma/index.htm).1A key component of this education is a written action plan. The EPR-3 recommends that clinicians provide all patients who have asthma with a written action plan that includes instructions for (1) daily management and (2) recognizing and handling worsening asthma, including adjustment of dose of medications. Action plans may be based on PEF measurements, symptoms, or both, depending on patient and clinician preference (Evidence B). If a patient has difficulty in recognizing the signs of worsening asthma, a peak flow- based plan may be particularly useful (Evidence D).
CONCLUSIONS
The new NAEPP guidelines recognize the variability of asthma and the need to focus on asthma control through periodic and ongoing assessment of current impairment, including symptoms, nighttime awakenings, SABA use, functional limitations, and the future risk of exacerbations or treatment-related adverse effects. Parents and children should have an active role in their asthma care. Written action plans and asthma self-management education are essential to create a partnership between patients and physicians for optimal asthma care. Physicians should encourage follow-up every 3 to 6 months for ongoing assessment of asthma control and the need to increase or reduce pharmacological treatment. Physicians should use guidelines for step-up and step-down therapy. As in the earlier iterations of the NAEPP guidelines, ICSs are still recommended as the preferred medication for initiating long-term control therapy in children of all ages. Importantly, new evidence supports the initiation of ICS therapy in young children at risk for asthma.1 *
References:
REFERENCES:
1.National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Bethesda, Md: National Heart, Lung, and Blood Institute; August 2007. NHLBI publication 08-4051. Available at: http://www.nhlbi.nih.gov/ guidelines/asthma/asthgdln.pdf. Accessed December 13, 2007.
2.National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. Bethesda, Md: National Heart, Lung, and Blood Institute; October 2007. NHLBI publication 08-5846.
3. Chipps BE, Spahn JD. What are the determinants of asthma control? J Asthma. 2006;43:567-572.
4. Chipps BE, Spahn JD, Sorkness CA, et al. Variability in asthma severity in pediatric subjects with asthma previously receiving short-acting b2-agonists. J Pediatr. 2006;148:517-521.
5. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133-138.
6. Castro-RodrÃguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
7. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354: 1985-1997.
8. Guilbert TW, Morgan WJ, Krawiec M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education Network. Control Clin Trials. 2004;25:286-310.
9. Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282-1287.
10. Zanconato S, Meneghelli G, Braga R, et al. Office spirometry in primary care pediatrics: a pilot study. Pediatrics. 2005;116:e792-e797.
11. Spahn JD, Chipps BE. Office-based objective measures in childhood asthma. J Pediatr. 2006;148: 11-15.
12. Asthma and Allergy Foundation of America (AAFA). Sleep/Work/Play and Sleep/Learn/Play resources. Available at: http://www.sleepworkplay. com/. Accessed October 5, 2007.
13. Childhood Asthma Control Test™ for Children With Asthma Aged 4 to 11 Years. © 2002, by QualityMetric Incorporated. Asthma Control Test is a trademark of QualityMetric Incorporated. Available at: http://www.asthmacontroltest.com/index.htm. Accessed October 7, 2007.
14. Bacharier LB, Phillips BR, Bloomberg GR, et al, for the Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604-610.
15. Bisgaard H, Allen D, Milanowski J, et al. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87-94.
16. Donahue JG, Weiss ST, Livingstone JM, et al. Inhaled steroids and the risk of hospitalization for asthma. JAMA. 1997;277:887-891.
17. Leflein JG, Szefler SJ, Murphy KR, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics. 2002;109:866-872.
18. Tasche MJ, Uijen JH, Bernsen RM, et al. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax. 2000;55:913-920.
19. Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108:754. Abstract e48.
20. Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315-322.
21. Bisgaard H, Gilles J, Groenewald M, et al, on behalf of an International Study Group. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999;160:126-131.
22. Szefler SJ, Eigen H. Budesonide inhalation suspension: a nebulized corticosteroid for persistent asthma. J Allergy Clin Immunol. 2002;109:730-742.
23. Roorda RJ, Mezei G, Bisgaard H, et al. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol. 2001;108: 540-546.
24 .Russell G, Williams DA, Weller P, et al. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol. 1995;75:423-428.
25. Johnston NW, Mandhane PJ, Dai J, et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007; 120:e702-e712.
26. Garcia-Garcia ML, Wahn U, Gilles L, et al. Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma: the MOSAIC study. Pediatrics. 2005;116: 360-369.
27. Ostrom NK, Decotiis BA, Lincourt WR, et al. Comparative efficacy and safety of low-dose fluticasone propionate and montelukast in children with persistent asthma. J Pediatr. 2005;147:213-220.
28. Sorkness CA, Lemanske RF Jr, Mauger DT, et al, for the Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64-72.
29. Szefler SJ, Phillips BR, Martinez FD, et al, for the Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233-242.
30. Reed CE, Offord KP, Nelson HS, et al. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. J Allergy Clin Immunol. 1998; 101:14-23.
31. Accolate [package insert]. Wilmington, Del: AstraZeneca, LP; 2004.
32. Masoli M, Weatherall M, Holt S, et al. Systematic review of the dose-response relation of inhaled fluticasone propionate. Arch Dis Child. 2004;89: 902-907.
33. Shapiro G, Bronsky EA, LaForce CF, et al. Dose-related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatr. 1998; 132:976-982.
34. Pauwels RA, Löfdahl CG, Postma DS, et al, for the Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337: 1405-1411.
35. Zimmerman B, D'Urzo A, Bérubé D. Efficacy and safety of formoterol Turbuhaler® when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol. 2004;37:122-127.
36. Bisgaard H. Effect of long-acting b2 agonists on exacerbation rates of asthma in children. Pediatr Pulmonol. 2003;36:391-398.
37. Verberne AA, Frost C, Duiverman EJ, et al. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. Am J Respir Crit Care Med. 1998;158:213-219.
38. Malone R, LaForce C, Nimmagadda S, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Ann Allergy Asthma Immunol. 2005;95:66-71.
39. Van den Berg NJ, Ossip MS, Hederos CA, et al. Salmeterol/fluticasone propionate (50/100 mg) in combination in a Diskuse inhaler (Seretidee) is effective and safe in children with asthma. Pediatr Pulmonol. 2000;30:97-105.
40. Simons FER, Villa JR, Lee BW, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138:694-698.
41. Suessmuth S, Freihorst J, Gappa M. Low-dose theophylline in childhood asthma: a placebo-controlled, double-blind study. Pediatr Allergy Immunol. 2003;14:394-400.
42. Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2003;(4):CD001186.
43. Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-Study). J Allergy Clin Immunol. 2002;109: 251-256.
44. Niggemann B, Jacobsen L, Dreborg S, et al, PAT Investigator Group. Five year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61: 855-859.
45. Chipps BE, Szefler SJ, Simons ER, et al, for the TENOR Study Group. Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma. J AllergyClin Immunol. 2007;119:1156-1163.
46. Leone FT, Fish JE, Szefler SJ, et al, for the Expert Panel on Corticosteroid Use. Systematic review of the evidence regarding potential complications of inhaled corticosteroid use in asthma. Collaboration of American College of Chest Physicians, American Academy of Allergy, Asthma, and Immunology, and American College of Allergy, Asthma, and Immunology. Chest. 2003;124:2329-2340.
47. The Childhood Asthma Management Program Research Group (CAMP). Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
48. Pauwels RA, Pedersen S, Busse WW, et al, on behalf of the START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003; 361:1071-1076.
49. Roux C, Kolta S, Desfougères J-L, et al. Long-term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics. 2003;111:e706-713.
50. Ip M, Lam K, Yam L, et al. Decreased bone mineral density in premenopausal asthma patients receiving long-term inhaled steroids. Chest.1994; 105:1722-1727.
51. Israel E, Banerjee TR, Fitzmaurice GM, et al. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med.2001;345: 941-947.
52. Suissa S, Baltzan M, Kremer R, et al. Inhaled and nasal corticosteroid use and the risk of fracture. Am J Respir Crit Care Med.2004;169:83-88.
53. Wong CA, Walsh LJ, Smith CJ, et al. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet.2000;355:1399-1403.
54. Khan Y, Tang Y, Hochhaus G, et al. Lung bioavailability of hydrofluoroalkane fluticasone in young children when delivered by an antistatic chamber/ mask. J Pediatr. 2006;149:793-797.
55. Todd GR, Acerini CL, Ross-Russell R, et al. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child.2002;87:457-461.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.
Itchy skin associated with sleep problems in infants
September 27th 2024A recent study presented at the American Academy of Pediatrics 2024 National Conference & Exhibition, sheds light on the connection between skin conditions and sleep disturbances in infants and toddlers, highlighting itchy skin as a significant factor, even in the absence of atopic