Update on Sexually Transmitted Diseases:Human Papillomavirus Infection
Adolescents are at high risk for HPV infection because they tend to become involved with multiple simultaneous sexual partners rather than forming monogamous relationships.
Adolescents are at high risk for HPV infection because they tend to become involved with multiple simultaneous sexual partners rather than forming monogamous relationships.
This is false. In many respects, adolescents are at high risk for contracting and transmitting any sexually transmitted infection--not just HPV. As with most STDs, the highest incidence of new cases of HPV occurs in adolescents. Why?
First, while adolescents and their partners generally do not have multiple sexual partners at one time, many do engage in "serial monogamy" (ie, a series of monogamous sexual relationships over time) instead of establishing long-term sexual relationships with a single partner.
Second, many teens have unprotected sex with a partner who has visible genital warts or who has a well-known history of HPV infection. This behavior may be the result of a lack of knowledge about how the virus is transmitted, or even a consequence of "emotional coercion" by the partner.
Third, when sexual activity is combined with inconsistent condom use (another hallmark and risk of adolescent sexual activity), the adolescent places himself or herself at repeated high-risk exposure to disease.
Adolescent girls, in particular, are at increased physiologic risk for cervical infection. The adolescent female's cervix has a significant degree of ectopy (exposed columnar epithelium rather than squamous epithelium) that places her at increased risk. As with most STDs, the virus can infiltrate the columnar epithelium with much greater ease than the squamous epithelial cells found in a mature cervix.
Even when condoms are used correctly and consistently, they do not provide adequate protection against HPV infection.
This is true. Genital warts and high-risk oncogenic HPV genotypes are transmitted via direct sexual contact with an infected partner. These genotypes can live on the penile shaft (especially the prepuce), scrotum, vulva, vagina, cervix, anus and, occasionally, in the oral mucosa. Transmission occurs when the infected body part comes into contact with a partner's genitals, anus, or mouth. Micro-abrasions sustained during sexual contact provide the access point for the virus to enter the epithelial cell layers, where it subsequently replicates and proliferates.
Consistent condom use decreases the risk of HPV transmission, but protection is far from complete. Condoms only provide protection if the infected area is effectively covered. Therefore, a male who has the virus on his scrotum may still transmit infection to a partner during sexual activity even if he wears a condom, because the condom does not cover the infected area.
Despite their shortcomings, condoms can offer protection. Adolescents need to be counseled that although condom use does not offer 100% protection, proper use will significantly decrease the transmission of all sexually transmitted infections--including HPV infection.
The warts found on a patient's hands or feet are caused by different genotypes of the same virus that causes genital warts or cervical cancer.
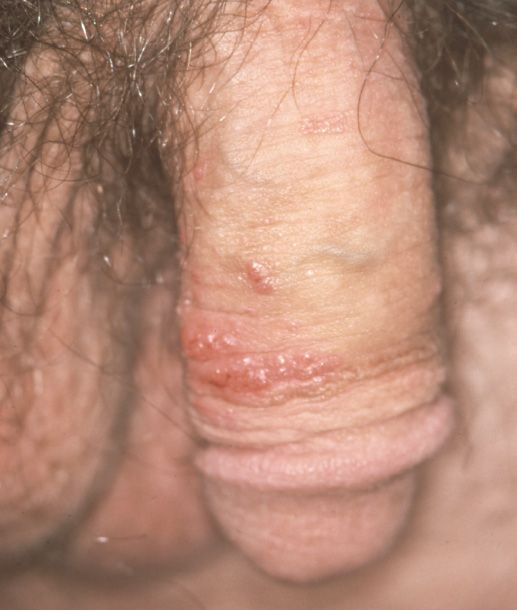
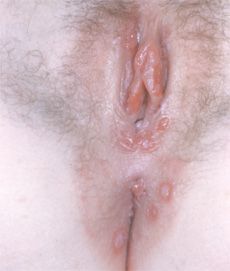
Figures – Human papillomavirus (HPV) types 6 and 11 have a predilection for the external genitalia, where they cause condylomata acuminata. These genotypes generally do not cause the cervical dysplastic changes associated with HPV types 16, 18, 31, 33, 35, 52, and 55.
This is true. HPV, a small double-stranded DNA virus, has over 100 genotypes with a multitude of different presentations. For example, in the general pediatric and adult populations, HPV types 1, 2, and 7 cause verrucae vulgaris and verrucae plantaris. HPV genotypes 6 and 11 are commonly associated with sexually transmitted condylomata acuminata (Figures) because they have a predilection for the external genitalia. For the most part, these genotypes are considered low risk because they typically do not induce dysplastic changes in the skin or mucosa that lead to neoplasms.
In contrast, HPV genotypes 16, 18, 31, 33, 35, 52, and 55 are strongly associated with cervical dysplasia, which--if untreated--can lead to cervical carcinoma. Infection with these genotypes frequently is asymptomatic, and the femaleadolescent is unaware of her infection until a Papanicolaou (Pap) smear reveals atypical cells. Therefore, it is important to counsel adolescent patients that the absence of visible genital warts does not preclude the possibility of infection with high-risk genotypic HPV.
Condylomata acuminata are primarily diagnosed by visual inspection rather than through biopsy testing.
This is true. Most cases of condylomata acumi- nata are diagnosed during visual inspection of the penis, vagina, anus, or cervix. The warts often resemble cauliflower, but they may appear fleshy, flat, or even keratinized.
If I see an unusual genital lesion and I am uncertain of the diagnosis, I apply a discrete amount of 3% acetic acid to the lesion. Genital warts often turn white and frothy--especially if they are located along the vulva or labia minora. I do not recommend "painting" the entire anogenital mucosa with acetic acid to find the warts: the acid will irritate the area and will likely cause false-positive findings (eg, lesions of psoriasis, herpes, and vaginitis may look similar when exposed to acetic acid).
Visual inspection for genital warts should include any area that might be exposed to a partner's genitals. If your patient engages in oral sex or receptive anal sexual contact, then the clinician should examine the mouth and anal area for the presence of warts. Anal warts can occasionally be found deep within the anorectal canal; anoscopy may occasionally be necessary to determine their presence.
A biopsy is usually not needed. However, to rule out a malignant finding it may be necessary to perform a biopsy on a lesion of unusual color or shape or one that is resistant to standardized treatments.
Once genital warts are present, they should be aggressively treated and removed as soon as possible to decrease the viral presence on the skin or mucosa and to reduce the risk of future outbreaks.
This is false. Many cases of genital warts resolve without any intervention. The prevailing theory is that the warts induce a localized humoral immunity at the site of infection. This results in spontaneous clearing within about a year of presentation. Therefore, when counseling adolescents about treatment options, remember that NOT treating may occasionally be a very reasonable plan.
In my experience, most adolescents are not willing to wait for resolution and demonstrate a strong preference for intervention. For many teenagers, the presence of genital warts elicits significant psychological distress. Occasionally, a condyloma causes functional impairment, especially if it is located around the urethra or in the anal area. Large warts in the anogenital area can bleed, smell foul, obstruct these outlets, and cause significant pain. Aggressive reduction may be warranted.
The typical case of genital warts can often be adequately treated at home, with minimal need for direct physician intervention and follow-up.
This is true. A variety of options are available once you and your patient have decided to initiate treatment. The clearance rates for these options are relatively comparable, although recurrence rates can vary depending on the treatment you choose. Remind your patients that wart removal does not immediately eradicate the underlying infection. Even after a wart is physically removed, the virus may remain active in the mucosa and can incite the proliferation of new lesions. This may occur until the body's humoral defenses remove the virus from the mucosa (at 12 to 24 months). Ultimately, new warts may emerge within that time that will require additional treatments.
Treatment options can be grouped into 3 broad categories:
•Patient-administered treatments.
•Office-based treatments.
•Surgical treatments.
Each option has pros and cons that should be discussed with the patient who needs to understand that his or her health insurance may not cover the cost of every option.
Patient-administered treatments include podofilox solution or gel and imiquimod cream.1 Their major benefit is that the patient can complete therapy at home, without needing to schedule weekly office visits.
Podofilox (0.5%) is a fairly inexpensive option that works by inhibiting condyloma cell mitosis. Imiquimod stimulates the local immune response and tends to be somewhat more effective than podofilox in both clearing the warts and preventing recurrences.3 However, it also tends to be more expensive. Both medications must be applied in a regimen of treatment cycles over 4 weeks to 3 months. If the adolescent fails to adhere to instructions, the result may be severe irritation, pain, and bleeding at the treatment site.
Office-based treatments include the application of bichloroacetic acid or trichloroacetic acid or cryotherapies (for example, liquid nitrogen).1 These treatments work via localized destruction of the cells. Acetic acid or cryotherapy preparations are applied weekly to the warts until resolution is achieved. Both modalities tend to induce resolution with a somewhat lower risk of recurrence than home-based therapies.
A major adverse effect of office- and home-based treatments is localized blistering, erythema, and pain at the areas surrounding the condyloma. To reduce the risk, one can first apply a layer of petroleum jelly to the mucosa around the wart in hopes of protecting the healthy area.
Surgical excision and/or carbon dioxide laser vaporization effectively remove warts in a single treatment.1 These interventions typically require the training and expertise of a dermatologist or surgeon; hence, they can be quite expensive. Recurrences often occur at the margins, and additional treatments may be needed. However, these may be the treatment of choice for larger lesions (such as Buschke-Lwenstein tumors) or for lesions on the inner aspect of the anorectal canal.
Factors such as diabetes, stress, and smoking increase the risk of severe, bulky genital warts (in low-risk HPV infection) and of cervical dysplasia (in high-risk HPV infection).
This is true. Immunocompromise for any reason (HIV infection, diabetes, or even significant psychological stress that affects the immune system) is considered a high-risk state for an adolescent with HPV infection. Giant condylomata (ie, Buschke-Lwenstein tumors) often develop in immunocompromised patients. If an immunocompromised person is infected with a high-risk HPV genotype, the risk of progression to cervical dysplasia increases significantly.
Tobacco use is a well-established risk factor for cervical cancer. The prevailing theory is that nicotine accumulates in cervical mucus and subsequently impairs the local humoral defenses against high-risk HPV. Although the body usually clears the HPV infection within 2 years, the risk of squamous cell carcinoma increases substantially if the body is unable to clear the infection within that period. Hence, the nicotine in cigarettes leads to prolonged HPV infection and an increased risk of squamous cell cervical carcinoma.
Other risk factors that may contribute to high-grade cervical neoplasia and cancer include multiple pregnancies, multiple sexually transmitted infections, oral contraceptive use, and having an uncircumcised male partner.4
Once an adolescent has genital warts, she is at high risk for cervical dysplasia.
This is false. As noted, genital condylomata usually result from exposure to HPV genotypes 6 and 11. These viral genotypes typically do not cause high-grade lesions. However, "low-risk" is not synonymous with "no-risk," and there still may be some potential for dysplastic cervical changes. More significant is the likelihood that the teen may have been co-infected with a high-risk genotype (such as 16 or 18).
Males with condyloma should be counseled that genital warts should not cause cervical cancer in their partner. However, concurrent high-risk HPV infection has cancerous potential if transmitted to a partner.
Many adolescents experience significant anxiety when genital warts are diagnosed--and are especially worried about the oncogenic possibility. While teenagers must take the diagnosis seriously, you can also remind them:
•The majority of HPV infections (with both low- and high-risk types) tend to be transient. Most resolve within 2 years.
•For the small percentage (fewer than 5%) of infected girls in whom cervical dysplasia develops, progression is extremely slow. If the patient undergoes appropriate Pap smear testing (see below), then chances are good that any dysplastic changes will be recognized early.
A 15-year-old girl confides to you that she has recently become sexually active (occasionally without condoms). In addition to counseling her about STDs and high-risk behavior, you advise her that she does not need to start annual Pap screening until she turns 18.
This is true. Pap smear screening (as well as the liquid-based cytology screens) evaluates the cervical transitional cells for dysplastic changes. The American College of Obstetricians and Gynecologists and the American Cancer Society recently adjusted their recommendations for Pap smear screening in adolescents.5-7 According to the new guidelines, annual Pap smear screening should be started 3 years after the patient begins having sexual intercourse or at age 21--whichever comes first. The rationale is to allow the opportunity for HPV infection to clear on its own rather than aggressively treating low-grade lesions that would otherwise self-resolve. Note: this recommendation only applies to adolescents with a competent immune system. Immunosuppressed patients need an annual Pap smear once they begin having sexual intercourse.
A few comments about Pap smear screening:
•Failure to obtain an adequate specimen is the most common reason for a repeated test. Therefore, Pap smear screening should be avoided while the patient is menstruating or demonstrates symptoms of cervicitis.
•Even though Pap smear screening can be delayed for 3 years after sexual debut, pelvic examination for other sexually transmitted infections should still be performed at least annually in a sexually active adolescent female.
Pap smears tell the clinician whether cervical cancer will develop, based on the presence of condylomatous cells and the number of cells undergoing mitosis at the time of the screening examination.
This is false. Pap smear results are essentially reported as normal or abnormal, depending on the presence of cellular atypia. The results serve as a screen for abnormalities rather than providing a true diagnosis. On the other hand, a diagnosis of cervical intraepithelial neoplasia or cervical cancer is based on specimens from a biopsy or colposcopy that are obtained after a patient has an abnormal Pap smear result.
The 2001 Bethesda Conference established the Pap smear reporting system that is widely used today.8 The reports consist of 3 sections: specimen adequacy, general categorization, and interpretation of results.
Specimen adequacy tells the clinician whether the specimen contains sufficient cervical cells to perform an analysis. General categorization indicates whether the results are normal. If the overall results are normal, the report essentially stops there. When an abnormality is present, the third section (interpretation of results) describes the abnormality more fully.
Not all abnormal results are caused by cellular abnormalities. Inflammation from infection, or even the presence of yeast or bacterial vaginosis can cause an abnormal interpretation. (These findings are usually well described in the report.) Frequently, reports describe "atypical squamous cells of undetermined significance" (ASC-US) or "atypical squamous cells; cannot exclude a high-grade squamous intraepithelial lesion" (ASC-H). These findings can represent anything from a transient cellular atypia to early cellular intraepithelial lesions.
Some fundamentals of management are described below.
A patient with ASC-US or ASC-H can be conservatively managed with repeated Pap smears over 6 to 12 months. She need not be immediately referred for colposcopic evaluation.
This is true. A review of the management of all the various abnormal Pap smear and colposcopy results is beyond the scope of this article. However, when a Pap smear reveals ASC-US or ASC-H, it is reasonable to repeat the examination in 4 to 6 months to see whether the atypia clears on its own. If the atypical squamous cells persist, then referral for colposcopy is indicated. If results of the repeated Pap smear screen are normal, then one more screen should be done in 4 to 6 months before the patient resumes an annual screening regimen. Alternatively, if you perform liquid-based cytology screenings, then a HPV genotype test result often will accompany any report of ASC. As long as the screen reveals a low-risk genotype, the patient can return to routine annual screening examinations.
SUMMARY
Up to 75% of all sexually active adolescents are infected with HPV. Male and female patients should be screened for genital warts during routine physical examinations. Sexually active females need counseling about the timing and initiation of Pap smear screening. In practice settings in which time or logistics preclude the performance of pelvic exams and Pap smears, it is prudent to develop solid working relationships with local gynecologists to whom patients can be referred for these evaluations.
A comprehensive set of screening and management guidelines for HPV infection can be obtained from the American Society for Colposcopy and Cervical Pathology at http://www.ascp.org/pdfs/ consensus/algorithms.pdf.
References:
REFERENCES:
1.
Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines 2002. Available at:
http://www.cdc.gov/STD/treatment/6-2002TG.htm#HumanPapillomavirusInfection
Accessed May 1, 2006.
2.
Koutsky LA, Kiviat NB. Genital human papillomavirus. In: Holmes KK, ed.
Sexually Transmitted Diseases.
3rd ed. New York: McGraw-Hill; 1999:347.
3.
Gunter J. Genital and perianal warts: new treatment opportunities for human papillomavirus infection.
Am J Obstet Gynecol.
2003;189(3 suppl):S3-S11.
4.
Castellsague X, Bosch FX, Munoz N. Environmental co-factors in HPV carcinogenesis.
Virus Res.
2002;89:191-199.
5.
Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer.
CA Cancer J Clin.
2002;52:342-362.
6.
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 61, April 2005. Human papillomavirus
. Obstet Gynecol.
2005;105:905-918.
7.
American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. ACOG Committee Opinion #300: cervical cancer screening in adolescents.
Obstet Gynecol.
2004;104:885-889.
8.
Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology.
JAMA.
2002;287:2114-2119.
Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.