Urine drug screens: Caveats for interpreting results
Rapid drug screening immunoassays quickly assess pediatric patients for drug exposure. However, certain limitations of these immunoassays call for caution when interpreting presumptive positive results.
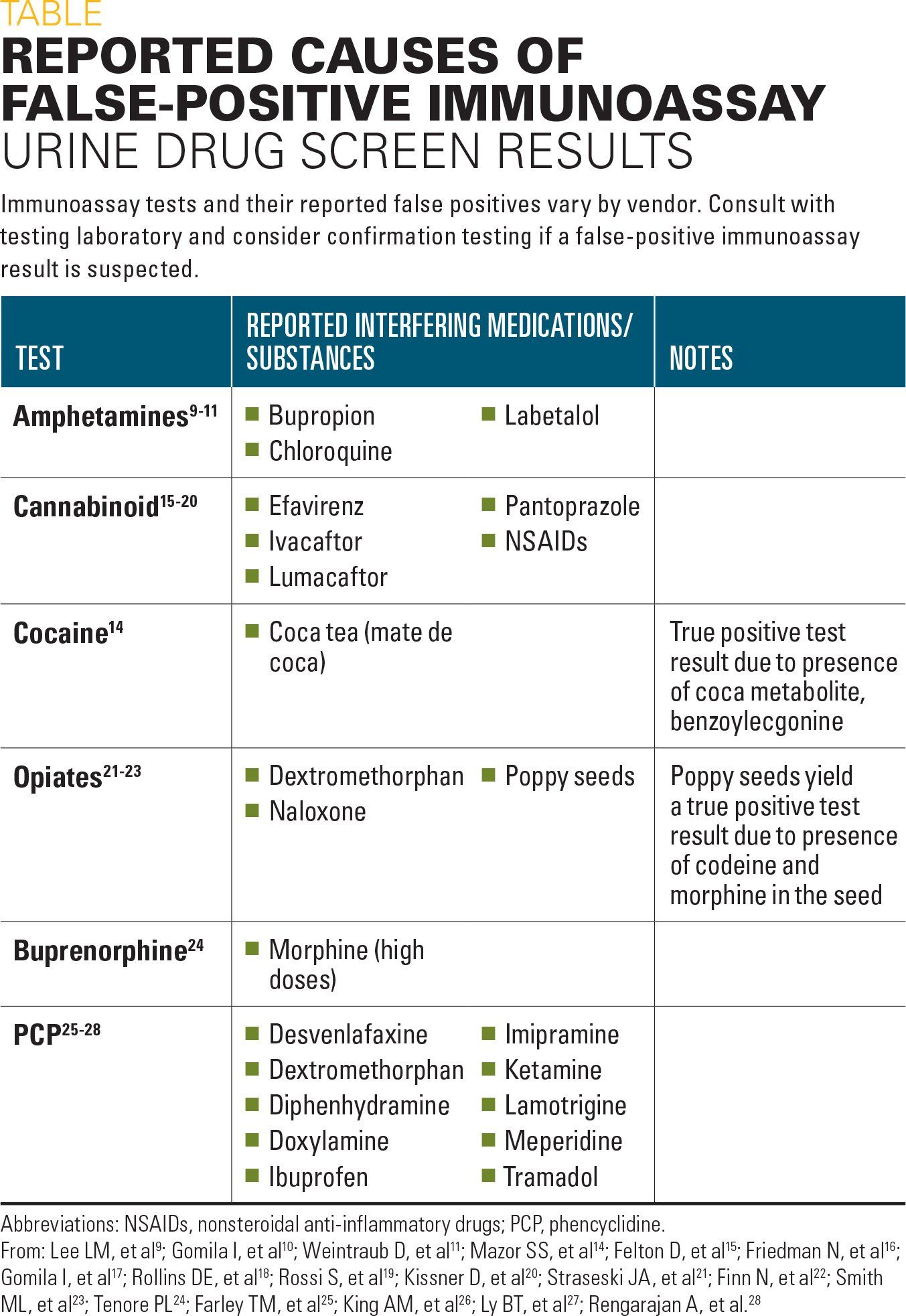
Table
Note from Dr Lee

Urine drug screens (UDS) have an integral role in the clinical management of pediatric patients.1,2 Pediatric Trauma Society guidelines recommend universal screening for all pediatric trauma patients aged older than 12 years,3 and substance abuse in the pediatric population is associated with increased injury severity, length of hospital stay, and mortality.4
Rapid screening methods, such as immunoassays, are commonly used in the emergent setting to screen patients for the presence of illicit drugs. These methods are advantageous because they can be readily automated and integrated into the workflow of high-throughput laboratories, providing a rapid turnaround time to enable timely patient management decisions.5 However, inherent limitations of UDS immunoassays necessitate caution when interpreting results.
Urine is the specimen of choice for UDS due to the increased window of drug detection compared with blood specimens and the relatively noninvasive nature of urine collection.6,7 The detection window represents the amount of time after drug administration a person continues to excrete the drug and/or metabolite at a concentration exceeding a cutoff level. The detection window (typically 1 to 3 days) can be influenced by several factors, including dose, route of administration, metabolism, urine concentration, pH, and lipophilicity.
Limitations of UDS immunoassays
Despite the many advantages of immunoassay-based UDS, considerable limitations should be noted. First, because the results are qualitative (positive or negative), the interpretation of UDS immunoassay results is based on cutoff values for each drug. Although the cutoff value is established by the manufacturer of each testing device and represents the quantitative number above which there is a high probability of detecting a drug if it is present, each laboratory may use different cutoff values.
All UDS immunoassays entail the binding of antibodies to drug molecules. However, the type of antibody employed can result in considerable differences in assay performance. Polyclonal antibodies generally detect a wide range of drugs within a particular class, but these antibodies also allow for more cross-reactivity with drugs outside this class, increasing the risk of false-positive results. In comparison, immunoassays using monoclonal antibodies detect fewer members of a drug class, thus reducing the likelihood of cross-reactivity with similar drugs outside the drug class. Additionally, positive results from class-specific immunoassays cannot be attributed to an individual drug, complicating interpretation.
The US Department of Health and Human Services (HHS) Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines recommend that initial drug screens test for the following commonly abused drugs or their metabolites: amphetamines, cocaine, marijuana (tetrahydrocannabinol/THC), opiates, and phencyclidine (PCP).8 Several studies have shown that UDS immunoassays for these drugs are subject to false-positive results (Table).
Reported causes of false-positive results
The major metabolite of labetalol can cause false-positive amphetamines UDS results.9 False-positive amphetamines UDS results in pregnant women given high-dose labetalol for the treatment of hypertensive disease have been reported.9 Chloroquine10 and bupropion11 also have been shown to cause false-positive amphetamines immunoassay results.
False-positive results from a cocaine UDS immunoassay have been reported. However, a definitive cause for the interference was not identified.12,13 Although it is not considered a cause of false-positive cocaine UDS results, coca tea (mate de coca) ingestion can cause positive cocaine results due to the presence of the coca metabolite benzoylecgonine, which is also a metabolite of cocaine.14
Pantoprazole, a commonly prescribed proton pump inhibitor (PPI) for the management of gastrointestinal (GI) symptoms, can cause false-positive test results with some cannabinoid immunoassay UDS.15-17 In addition to pantoprazole, nonsteroidal anti-inflammatory drugs and the antiretroviral drug efavirenz have been implicated in false-positive cannabinoid UDS immunoassays.18,19 Recently, the combination therapy lumacaftor/ivacaftor, which is used in a subset of cystic fibrosis patients, was identified as a cause of false-positive cannabinoid screens.20
Naloxone, which is recommended by the American Academy of Pediatrics (AAP) for the treatment of pediatric opioid overdose, cross-reacts with one type of opiate UDS immunoassay.21 Dextromethorphan exhibits a growing trend of abuse potential in adolescents and it cross-reacts with an assay for opiates, causing false-positive results.22 Another opiate-testing caveat is that poppy seed ingestion can cause a positive result on opiate UDS immunoassays, due to the presence of morphine and codeine in the seeds.23 Conversely, high-dose morphine can cause a false positive in the UDS immunoassay for buprenorphine, an opiate-addition medication.24
Although PCP abuse has declined recently, PCP remains a common component of UDS testing.8 False-positive PCP UDS immunoassay results can be caused by several drugs, including dextromethorphan, ibuprofen, imipramine, meperidine, ketamine, lamotrigine, and tramadol.25-28
False-negative UDS immunoassay results
Another limitation of UDS immunoassays is the possibility of unexpected and false-negative results. A strict interpretation of a negative UDS result is that at the time of specimen collection, the concentrations of those drugs for which a test was performed were less than the threshold limits required to call the test positive. For drug-class UDS assays, antibody cross-reactivity can vary considerably within the drug class, leading to unexpected negative results. For example, many opiate UDS immunoassays have little-to-no cross-reactivity with fentanyl.
Caveats when interpreting positive results
Because of the limitations of UDS immunoassays described above, positive UDS results should be interpreted as “presumptive positive” results, and, if definitive results are required, confirmatory testing by mass spectrometry should be conducted. The protocol for screening and confirmation differs between laboratories depending on clinical needs, test menu, methodology employed, and the testing capability of the laboratory. Interpretive result comments and consultation with a laboratory director are valuable approaches to aid the accurate interpretation of UDS immunoassay results and other toxicology testing.
Positive UDS immunoassay results do not provide sufficient information to determine the exposure time, dose, or the frequency or pattern of drug use.29 Among the many variables to be considered when interpreting UDS immunoassay results are drug-drug interactions, assay cross-reactivity, drug formulation impurities, urine adulteration, genetic variation in drug-metabolizing enzymes, dose management, and drug half-life. Misleading but true-positive results can be obtained. Knowledge of the potential causes of false-positive and false-negative UDS immunoassay results is critical when determining the appropriate course of action in the context of patient care.
References:
1. Christian MR, Lowry JA, Algren DA, Thornton SL, Deng S, and Garg U. Do rapid comprehensive urine drug screens change clinical management in children? Clin Toxicol (Phila). 2017;55(9):977-980.
2. Hoffman RJ, Nelson L. Rational use of toxicology testing in children. Curr Opin Pediatr. 2001;13(2):183-188.
3. Nicolson NG, Lank PM, Crandall ML. Emergency department alcohol and drug screening for Illinois pediatric trauma patients, 1999 to 2009. Am J Surg. 2014;208(4):531-535.
4. Martin KL, Vogt KN, Girotti MJ, Stewart TC, Parry NG. Drug use and screening in pediatric trauma. Ther Drug Monit. 2011;33(4):439-442.
5. Melanson SE, Lee-Lewandrowski E, Griggs DA, Long WH, Flood JG. Reduced interference by phenothiazines in amphetamine drug of abuse immunoassays. Arch Pathol Lab Med. 2006;130(12):1834-1838.
6. Caplan YH, Goldberger BA. Alternative specimens for workplace drug testing. J Anal Toxicol. 2001;25(5):396-399.
7. Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage. 2004;27(3):260-267.
8. Substance Abuse and Mental Health Services Administration (SAMHSA). Clinical drug testing in primary care. Technical Assistance Publication (TAP) 32. HHS Publication No. (SMA) 12-4668. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012:1-96. Available at: https://store.samhsa.gov/system/files/sma12-4668.pdf. Accessed May 1, 2019.
9. Yee LM, Wu D. False-positive amphetamine toxicology screen results in three pregnant women using labetalol. Obstet Gynecol. 2011;117(2 pt 2):503-506.
10. Gomila I, Quesada L, Lopez-Corominas V, et al. Cross-reactivity of chloroquine and hydroxychloroquine with DRI amphetamine immunoassay. Ther Drug Monit. 2017;39(2):192-196.
11. Weintraub D, Linder MW. Amphetamine positive toxicology screen secondary to bupropion. Depress Anxiety. 2000;12(1):53-54.
12. De Giovanni N, Fucci N. Hypothesis on interferences in kinetic interaction of microparticles in solution (KIMS) technology. Clin Chem Lab Med. 2006;44(7):894-897.
13. Kim JA, Ptolemy AS, Melanson SE, Janfaza DR, Ross EL. The clinical impact of a false-positive urine cocaine screening result on a patient’s pain management. Pain Med. 2015;16(6):1073-1076.
14. Mazor SS, Mycyk MB, Wills BK, Brace LD, Gussow L, Erickson T. Coca tea consumption causes positive urine cocaine assay. Eur J Emerg Med. 2006;13(6):340-341.
15. Felton D, Zitomersky N, Manzi S, Lightdale JR. 13-year-old girl with recurrent, episodic, persistent vomiting: out of the pot and into the fire. Pediatrics. 2015;135(4):e1060-e1063.
16. Friedman N, Gantz J, Finkelstein Y. An (un)fortune cookie: a 2-year-old with altered mental status. Pediatr Emerg Care. 2017;33(12):811-814.
17. Gomila I, Barceló B, Rosell A, Avella S, Sahuquillo L, Dastis M. Cross-reactivity of pantoprazole with three commercial cannabinoids immunoassays in urine. J Anal Toxicol. 2017;41(9):760-764.
18. Rollins DE, Jennison TA, Jones G. Investigation of interference by nonsteroidal anti-inflammatory drugs in urine tests for abused drugs. Clin Chem. 1990;36(4):602-606.
19. Rossi S, Yaksh T, Bentley H, van den Brande G, Grant I, Ellis R. Characterization of interference with 6 commercial delta9-tetrahydrocannabinol immunoassays by efavirenz (glucuronide) in urine. Clin Chem. 2006;52(5):896-897.
20. Kissner D, LeFlore Y, Narayan SB, Marigowda G, Simard C, Le Camus C. False-positive cannabinoid screens in adult cystic fibrosis patients treated with lumacaftor/ivacaftor. J Cyst Fibros. 2018;17(6):e51-e53.
21. Straseski JA, Stolbach A, and Clarke W. Opiate-positive immunoassay screen in a pediatric patient. Clin Chem. 2010;56(8):1220-1223.
22. Finn N, Wolf J, Louie J, Su B. High concentrations of dextromethorphan result in false-positive in opiate immunoassay test. Clin Chim Acta. 2015;448:247.
23. Smith ML, Nichols DC, Underwood P, et al. Morphine and codeine concentrations in human urine following controlled poppy seeds administration of known opiate content. Forensic Sci Int. 2014;241:87-90.
24. Tenore PL. False-positive buprenorphine EIA urine toxicology results due to high dose morphine: a case report. J Addict Dis. 2012;31(4):329-331.
25. Farley TM, Anderson EN, Feller JN. False-positive phencyclidine (PCP) on urine drug screen attributed to desvenlafaxine (Pristiq) use. BMJ Case Rep. 2017;2017:pii: bcr-2017-222106.
26. King AM, Pugh JL, Menke NB, Krasowski MD, Lynch MJ, Pizon AF. Nonfatal tramadol overdose may cause false-positive phencyclidine on Emit-II assay. Am J Emerg Med. 2013;31(2):444.e5-444.e9.
27. Ly BT, Thornton SL, Buono C, Stone JA, Wu AH. False-positive urine phencyclidine immunoassay screen result caused by interference by tramadol and its metabolites. Ann Emerg Med. 2012;59(6):545-547.
28. Rengarajan A, Mullins ME. How often do false-positive phencyclidine urine screens occur with use of common medications? Clin Toxicol (Phila). 2013;51(6):493-496.
29. Schwartz RH. Testing for drugs of abuse: controversies and techniques. Adolesc Med. 1993;4(2):353-370.

Anger hurts your team’s performance and health, and yours too
October 25th 2024Anger in health care affects both patients and professionals with rising violence and negative health outcomes, but understanding its triggers and applying de-escalation techniques can help manage this pervasive issue.