A case of late-onset group B Streptococcus infection in fraternal twins
A 29-year-old White woman presented to the labor and delivery unit due to preterm premature rupture of membranes and delivered twins. The twins were transferred to the neonatal intensive care unit following delivery.
The case
A 29-year-old White woman presented to the labor and delivery unit due to preterm premature rupture of membranes (PPROM). Gestational age was 32 weeks and 2 days by first trimester ultrasound, which correlated with her last menstrual period. That ultrasound also confirmed dichorionic diamniotic twin pregnancy.
After delivery (Table 1), the infants were transferred to the neonatal intensive care unit (NICU) because of apnea and were started on caffeine. Sepsis rule-out with 48 hours of ampicillin and gentamicin was given until blood cultures were reported negative. Maternal Group B streptococcus (GBS) screen done on admission returned a negative result.
Table 1
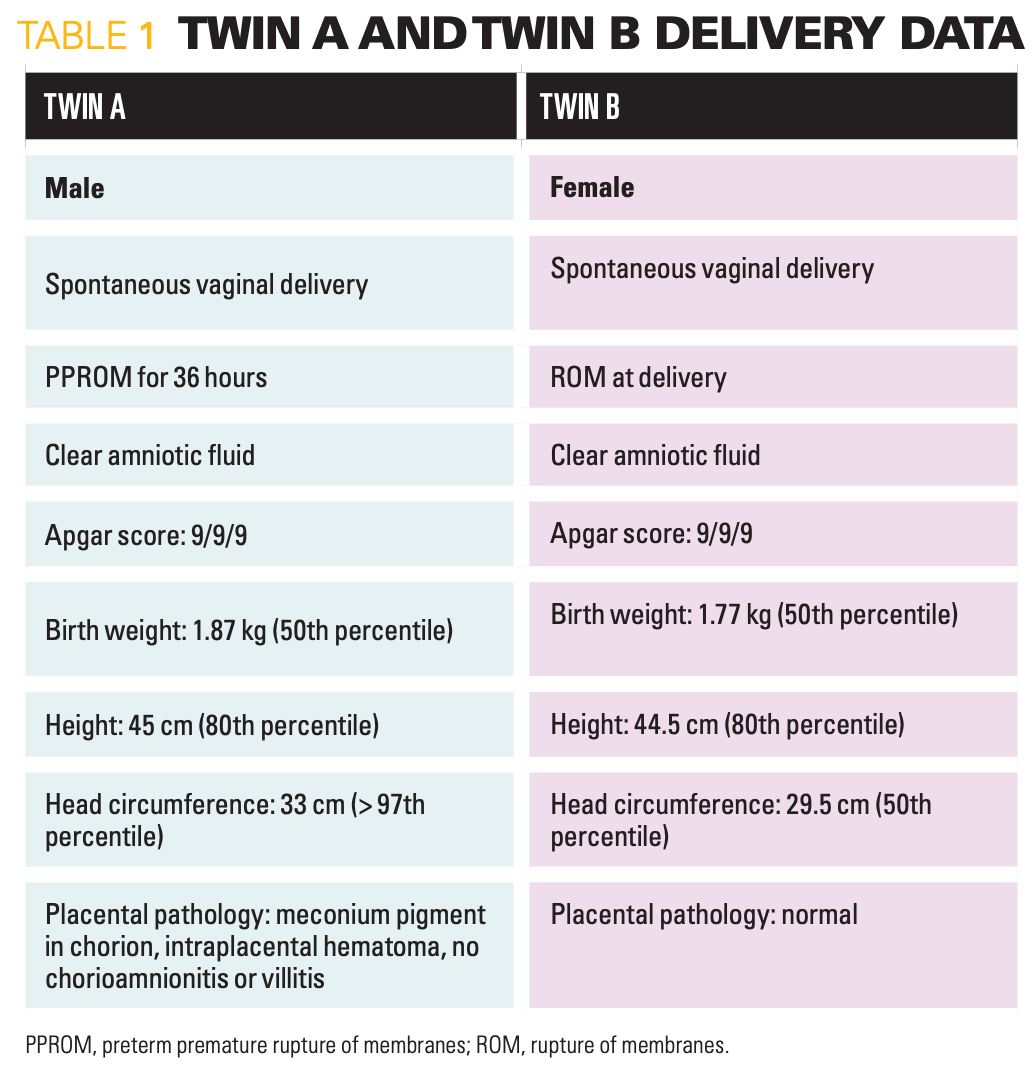
Twin A had a head ultrasound (HUS) on day of life (DOL) 1 due to macrocephaly. It revealed right grade 1 intraventricular hemorrhage (IVH). Twin B had a routine HUS on DOL 3, with normal results. Both Twins were treated with phototherapy for indirect hyperbilirubinemia of prematurity. They were transferred to the NICU-B, a step-down unit on DOL 3, and were progressing as expected.
Both babies were in the process of being discharged on DOL 23 when their mother noticed that Twin A was not breastfeeding well. On physical examination, he was pale, lethargic, and ill-appearing. His vital signs were significant for hypothermia of 35.7°C, tachycardia to 211 beats per minute (bpm), and low blood pressure of 40/15 mmHg. A lab work-up was done immediately. An intravenous (IV) access was secured for fluids and empirical antibiotics, with oxacillin and tobramycin given for 1 dose and then the patient was switched to vancomycin and cefepime.
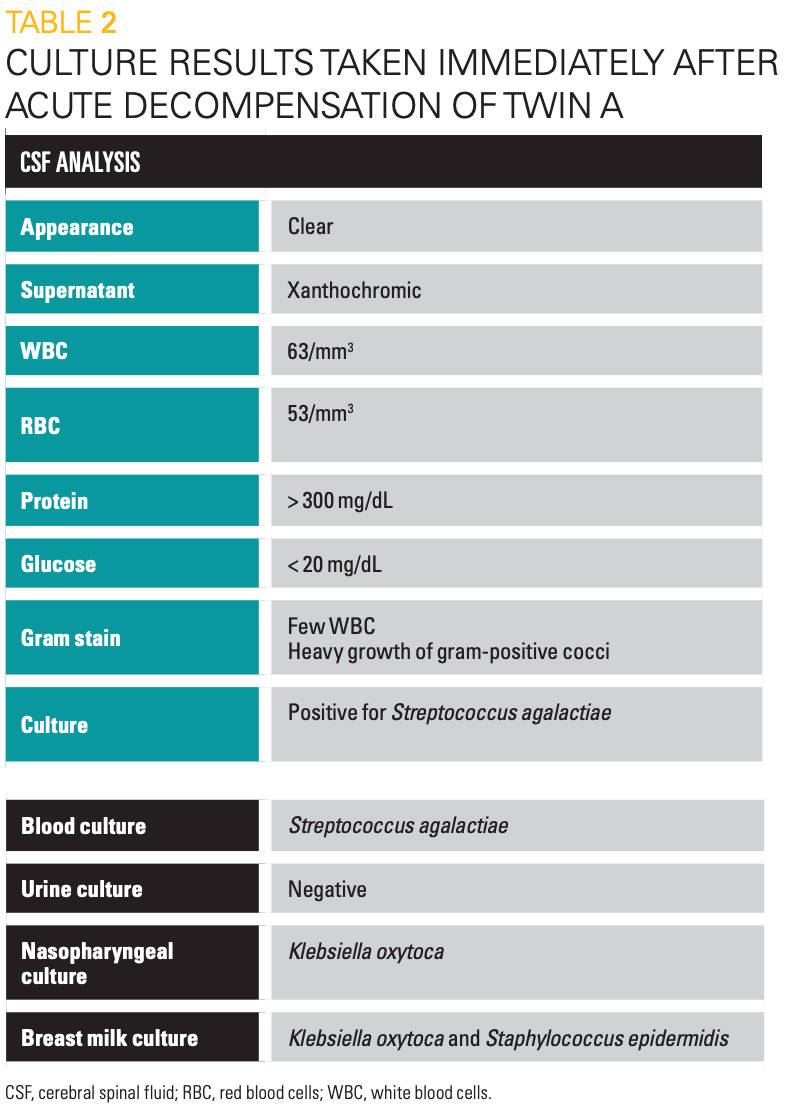
Twin A was transferred to NICU-A, the highest acuity unit, where he continued to get worse. Blood and cerebral spinal fluid (CSF) cultures resulted positive for Streptococcus agalactiae, GBS (Table 2). He was confirmed to have GBS meningitis and sepsis; antibiotics were appropriately switched to penicillin G with gentamicin for synergy. Septic shock and disseminated intravascular coagulation symptomatology ensued. He required ample pressure support and multiple transfusions of packed red blood cells (PRBCs), platelets, and fresh-frozen plasma, as well as a bicarbonate infusion to correct for acidosis. He then developed intractable seizures confirmed on electroencephalogram (EEG), requiring multiple antiseizure medications. Repeat HUS showed evolving right grade 1 IVH. Eventually, he required intubation and mechanical ventilation.
Table 2
During this time, consultations were made with pediatric infectious disease, neurology, and palliative care teams. Given his prognosis, a family conference was held. His parents were adamant that they did not want their baby to suffer anymore. A decision was made not to increase care beyond current support. The parents were allowed to hold their son. He died on DOL 25, approximately 48 hours after his acute decompensation.
What about Twin B?
Twin B remained stable and continued to feed well. She was without any signs or symptoms of sepsis and was well-appearing. However, due to the sudden deterioration of her Twin, a complete blood count (CBC) and C-reactive protein (CRP) were performed and were within normal limits. Her newborn screening test was within normal limits, and she passed the hearing screen. She was discharged on DOL 23 as previously planned and appropriate anticipatory guidance was given to both parents.
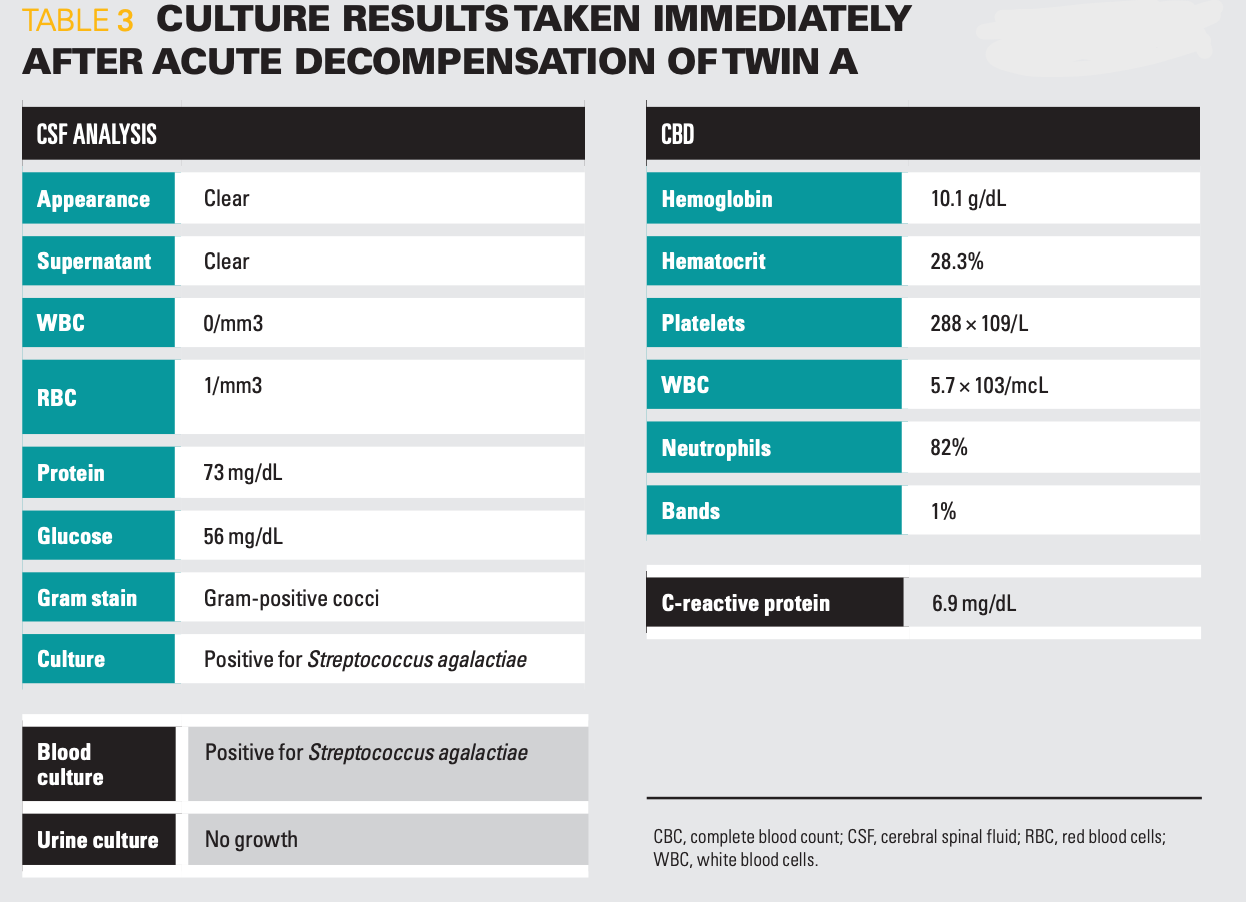
Approximately 4 days after hospital discharge at DOL 29, the mother presented with Twin B to the emergency department (ED) after she seemed more tired than usual with approximately a 5 mL decrease in oral intake per feed and was making grunting noises. On arrival, her vital signs were temperature of 37.2°C, heart rate of 174 bpm, blood pressure of 88/51 mmHg, respiratory rate of 54 breaths per minute, and 100% oxygen saturation on room air. A work-up was performed, including CBC, blood, urine, and CSF cultures (Table 3). Intravenous ampicillin and gentamicin were given empirically, and she was admitted to the pediatric floor.
Table 3
Hospital course for Twin B
She was admitted from the ED for suspected GBS meningitis and sepsis, which was subsequently confirmed. Pediatric infectious disease was consulted and recommended treatment for 14 days with meningitic doses of ampicillin every 6 hours with 48 hours of gentamicin on-board for a synergistic effect. Her repeat HUS was normal and repeat blood culture after 48 hours of antibiotics and on day 7 of admission were negative. The CSF culture was not repeated based on her clinical status. She was well-appearing throughout her hospital course and was gaining weight at approximately 35 g per day and developing appropriately. Her initial inflammatory marker, CRP of 6.9 mg/dL, declined to 1.2 mg/dL by day 12 of antibiotics. She was discharged home after approximately 2 weeks, now DOL 44, with a follow-up appointment with the pediatrician in 48 hours and audiology.
Return of Twin B
Six days after hospital discharge, on DOL 50, she returned to the hospital for increased fussiness and irritability, which had started approximately 9 hours before arrival to the ED. Her parents said she was feeling warm, but her rectal temperature was 37.7°C at home. Her parents gave her simethicone, but the fussiness and irritability continued. They brought her for evaluation given the prior history of GBS meningitis and recent discharge.
In the ED, vital signs were significant: febrile to 38.5°C, tachycardia at 184 bpm; blood pressure 97/46 mmHg; and tachypnea rate 64 breaths per minute with 100% oxygen saturation on room air. She received two 20 cc/kg saline boluses and acetaminophen with improvement in vital signs. Lab work-up included CBC, comprehensive metabolic panel (CMP), urine, and blood culture. Lumbar puncture (LP) was attempted 4 times in the ED, but the team was unable to obtain CSF. Given the recent history of GBS bacteremia and meningitis, she received meningitic doses of ampicillin and ceftriaxone empirically and was transferred to the floor for further work-up and treatment. Once on the floor, CSF was obtained for culture and chemistry.
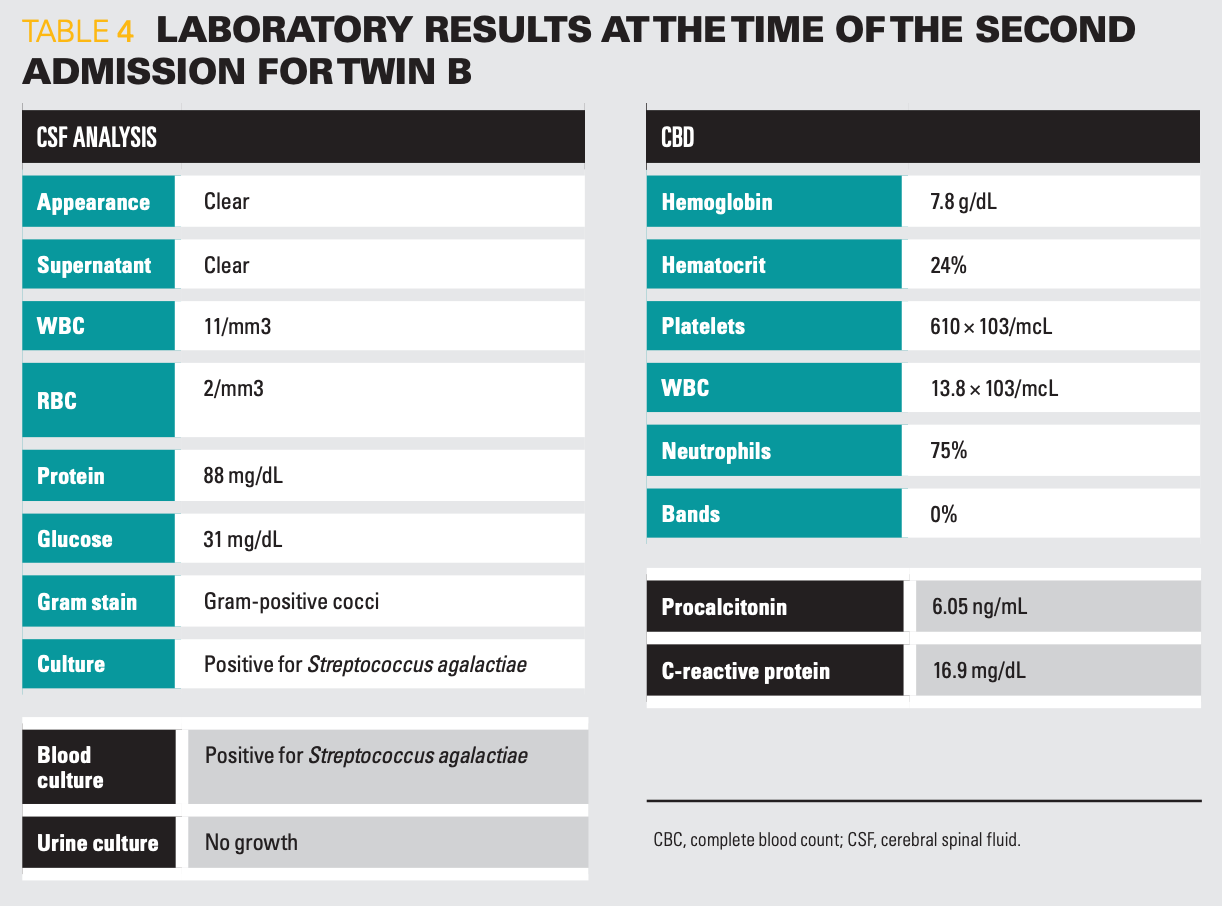
She had recurrent GBS meningitis and bacteremia (Table 4).
Table 4
Pediatric infectious disease was once again consulted. Recommendations were given as follows:
- Treatment with ampicillin and ceftriaxone for 21 days after cultures are negative
- Susceptibility testing of GBS isolates to ampicillin
- Reculturing of maternal breast milk
- Echocardiogram for vegetations
- HUS and brain magnetic resonance imaging
- Repeat audiology testing
- Rifampin treatment for 4 days after completion of therapy to eradicate potential mucosal colonization
She was given IV ceftriaxone for the duration of treatment via a peripherally inserted central catheter line. She was transfused with 10 cc/kg of PRBCs for increasing tachycardia without increasing inflammatory markers or clinical deterioration. This anemia was likely iatrogenic due to frequent blood sampling. After discussing the results with parents, they decided to start feeding her formula. She was also being seen by physical therapy, child life, and nutrition. She showed good response to therapy and continued to gain weight and develop adequately. She was discharged on DOL 75 with discharge medications, including rifampin, for 4 days to treat oral mucosal colonization from breast milk and daily multivitamin with iron. Follow-up appointments were made with her pediatrician, audiology, cardiology, neurology, and physical therapy.
Discussion
GBS is a major cause of neonatal morbidity and mortality as it remains one of the leading causes of neonatal sepsis and meningitis.1 GBS is a gram-positive diplococcus, a common colonizer of the gastrointestinal and genital tracts in pregnant women, and this colonization is often asymptomatic in pregnancy.2
The age of onset classifies GBS in neonates as follows1:
- Early-onset GBS presents from birth through DOL 6 but generally presents within the first 24 hours after delivery.3
- Late-onset GBS usually occurs at 4 to 5 weeks of life and may present from DOL 7 to 3 months of life.4
The most common pathogenesis of early-onset GBS infection is vertical transmission by ascending colonization from the maternal gastrointestinal and genitourinary flora. Then infection occurs with subsequent colonization and invasion of the fetal compartment or fetal aspiration of infected amniotic fluid.5 This transmission primarily occurs shortly before or during labor and delivery. Late-onset GBS infection may be acquired vertically as well, but horizontal transmission from colonized household contacts, hospital contacts, and breast milk is also a practical pathogenesis.6
The incidence of early-onset GBS has declined in the United States, and this is predominantly because of maternal GBS screening and intrapartum antibiotic prophylaxis (IAP), which was done in this case. In contrast, the incidence of late-onset GBS disease has remained approximately unchanged over the past 30 years.2 This is not surprising as IAP is only effective during labor and delivery and does not necessarily eradicate GBS colonization.7 Thus, even after women are given IAP, they may continue to be GBS colonized postpartum.8
Twin A had rapid clinical deterioration after meeting discharge criteria 48 hours prior to his death. He had profound septic shock and meningitis with intractable clinically observed seizures despite multiple medications. Although HUS was done immediately and repeated during his acute decompensation, an early MRI may have provided more details and possible pathology. In hindsight, perhaps the macrocephaly diagnosed prenatally on obstetric ultrasound played a more significant role in his disease susceptibility, prognosis, rapid progression, and eventual mortality.
Results of work-up

Horizontal transmission from colonized contacts plays a significant role in the pathogenesis of late-onset GBS, whether from parents or hospital staff. In this case it remains a likely possibility, especially given that maternal GBS screen resulted negative, IAP was administered, and both Twins had prolonged hospitalization from birth to DOL 23 before clinical deterioration. It can even be hypothesized that maternal breast milk was the agent of horizontal transmission.
An important clinical question has been raised about treating Twins and other multiples. What should be done when invasive GBS infection occurs in 1 sibling but the other sibling/siblings remain (or appear to remain) asymptomatic?
When invasive GBS infection occurs in an infant, the other Twin or multiples should be observed carefully for signs of infection.1 Whether this observation should be in hospital and for what duration or with the parents after thorough anticipatory guidance and counseling is yet to be determined.
The unaffected sibling/siblings likely may be colonized with GBS, given shared contacts and medical history, but there is no evidence to support a full antibiotic course in the absence of confirmed GBS disease.9
However, some experts take a conservative approach of empirical evaluation and antibiotic therapy even in the asymptomatic sibling/siblings when the index case has invasive GBS disease.10 This then leads to the discussion of adequate duration of treatment when there is no identified source of infection in the asymptomatic sibling/siblings.
Another important takeaway from this case is the importance of prompt CSF testing for sterility after antibiotic treatment, despite the patient’s clinical appearance.
DIFFERENTIAL DIAGNOSIS FOR LATE-ONSET SEPSIS IN PRETERM INFANTS
- Staphylococcus aureus and coagulase-negative staphylococci
- Other gram-negative organisms: Escherichia coli, Klebsiella, Pseudomonas, Enterobacter, Citrobacter, and Serratia
- Candida infections
- Herpes simplex virus
Patient outcome
Follow-up with Twin B at 15 months revealed that she was developmentally appropriate. Her vision and hearing were normal. EEG and brain MRI at age 1 year were normal. She qualified for early intervention based on her prematurity and significant course of meningitis and currently attends physical therapy and occupational therapy.
Future directions
There are limitations to GBS screening and IAP, especially because the exact delivery time cannot be predicted, particularly in the premature population. Universal antenatal testing of pregnant women for GBS colonization is obtained at 36 to 37 6/7 weeks gestation and on presentation in all preterm pregnancies.11 If administered to pregnant women and all sexually active females, the GBS vaccine is a promising solution that could potentially prevent these infections, especially late-onset disease. Clinical trials are being done on multivalent glycoconjugate GBS vaccines.12 Vaccine prevention remains a better solution than disease management.
Conclusion
This case serves as a reminder of the critical importance of prompt evaluation and empirical treatment of neonates at risk of invasive bacterial infection. There is an increased relative risk of invasive GBS infection in the Twin or other multiples of an affected patient, and therefore clinical hypervigilance must be maintained.
In invasive GBS disease, especially GBS meningitis, response to therapy and potential complications must be monitored clinically through serial neurologic examinations, repeat CSF analysis, and neuroimaging. In this patient with recurrent GBS meningitis, there is a high risk of permanent neurological sequelae, which so far, the patient has seemingly avoided.
References
1. American Academy of Pediatrics. Group B streptococcal infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS eds. Red Book: 2018 Report of the Committee on Infectious Diseases, 31st ed. American Academy of Pediatrics, 2018.
2. Puopolo K, Lynfield R, Cummings J, et al. Management of infants at risk for Group B streptococcal disease. Pediatrics. 2019;144(2),e20191881. doi: 10.1542/peds.2019-1881.
3. Eichenwald EC. Perinatally transmitted neonatal bacterial infections. Infect Dis Clin North Am. 1997;11(1):223-39. doi: 10.1016/s0891-5520(05)70350-0.
4. Puopolo, K. and Baker, C. Group B streptococcal infection in neonates and young infants. Updated October 12, 2021. Accessed December 6, 2021. https://www.uptodate.com/contents/group-b-streptococcal-infection-in-neonates-and-young-infants?search=group-b-streptococcal-infection-in-neonates-and-younginfants&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
5. UptoDate. Group B streptococcus. Accessed December 6, 2021. https://www.uptodate.com/contents/group-b-streptococcal-infection-in-neonates-and-young-infants/abstract/7,8
6. Morinis J, Shah J, Murthy P, Fulford M. Horizontal transmission of group B streptococcus in a neonatal intensive care unit. Paediatr Child Health. 2011;16(6): e48-50. doi:10.1093/pch/16.6.e48
7. Lin, F-Y, Weisman L, Azimi P, et al. Assessment of intrapartum antibiotic prophylaxis for the prevention of early-onset group B Streptococcal disease. Pediatr Infect Dis J. 2011; 30(9): 759-63. doi:10.1097/INF.0b013e31821dc76f
8. Patras K, Nizet V. Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front Pediatr. 2018; 6(27). doi:10.3389/fped.2018.00027
9. American College of Obstetricians & Gynecologists. Prevent of group B streptococcal early-onset disease in newborns. Published January 3, 2020. Accessed December 6, 2021. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns.pdf
10. Doran K, Benoit V, Gertz R, Beall B, Nizet V. Late-onset group B streptococcal infection in identical twins: insight to disease pathogenesis. J Perinatol. 2002;22(4): 326–330. doi: 10.1038/sj.jp.7210675
11. Morgan JA, Zafar N, Cooper DB. Group B streptococcus and pregnancy. In: StatPearls https://www.ncbi.nlm.nih.gov/books/NBK482443/
12. ClinicalTrials.gov. Group B streptococcus vaccine in healthy females - study results. Updated February 2, 2021. Accessed December 6, 2021. https://clinicaltrials.gov/ct2/show/results/NCT03807245
