Alopecia in adolescents
Hair loss is a condition that is often distressing and difficult to treat, particularly in adolescents. Here’s what you should know.
Alopecia in adolescents | Image Credit: © Bongkochrut - © Bongkochrut - stock.adobe.com.

Most cases of pediatric alopecia are acquired; the causes are heterogeneous and include nutritional, autoimmune, infectious, hormonal, and traumatic etiologies (Table 1). Alopecia can be classified by relative disease extent (eg, focal, patterned, generalized) and based on hair follicle preservation (nonscarring) or destruction (scarring). If left untreated, long-standing inflammation can damage the hair follicle, leading to permanent hair loss. Hair plays a vital role in identity formation, and the impact of alopecia on adolescents extends well beyond physical appearance.1 Consideration of an adolescent’s emotional and psychosocial well-being is imperative. This article reviews a few common and underrecognized causes of hair loss in adolescents and offers a practical approach to diagnosis and holistic management, including psychological considerations.
Androgenetic alopecia
Although androgenetic alopecia (AGA) is well recognized in adults, it is underrecognized in adolescents. In a large, single-center retrospective review, 13% of 438 pediatric patients (aged 8-19 years) seeking care for hair loss were diagnosed with AGA, representing the second most common type of hair loss behind alopecia areata.2 AGA classically presents with thinning of hair at the vertex and bitemporal regions in men. A female pattern of AGA presents with thinning and widening of the middle part (Ludwig pattern).3 Notably, adolescent boys can present with a female pattern.2
Systemic signs of hyperandrogenism, including hirsutism, acne, seborrhea, and polycystic ovary syndrome (PCOS; in adolescent girls), and a strong family history of AGA can support the diagnosis.2,3 In prepubertal children, androgen levels may be developmentally appropriate, and AGA typically presents around adrenarche (aged 5 to 8 years).3
AGA is primarily a clinical diagnosis and can be supported by trichoscopy showing greater than 20% hair diameter variability due to hair follicle miniaturization.4 A complete hormonal workup or endocrinology referral should be considered when there is concern for an underlying endocrine disorder (eg, in patients with PCOS, abnormal pubertal development, or other signs of metabolic syndrome).2,3,5
There are no approved treatments for AGA in children. Topical minoxidil (2% or 5% foam or solution) is sometimes used off-label. The topical foam may be a better option for children due to a lower risk for systemic absorption. Parents of younger children should be counseled that oral ingestion can cause low blood pressure.6 Local irritation, itch, and hypertrichosis are common adverse effects.3
Low-dose oral minoxidil (LDOM), either as monotherapy or in combination with other topical (eg, minoxidil, 5a-reductase inhibitors) or oral treatments (eg, spironolactone), is a newly explored therapy for children with AGA.7,8 Optimal pediatric dosing has not been established and ranges from 0.25 to 0.5 mg per day.7,8 De Nicolas-Ruanes et al7 reported clinical improvement in 80% of patients (aged 10-17 years) with adverse effects, ranging from facial hypertrichosis (most common) to hair shedding and hypotension in a minority of patients; all adverse effects were managed with dose adjustments. A combination of LDOM with spironolactone may mitigate hypertrichosis.8

Alopecia areata
Figure 1. On the scalp, there is a smooth and well-demarcated patch of complete hair loss, representing alopecia areata.
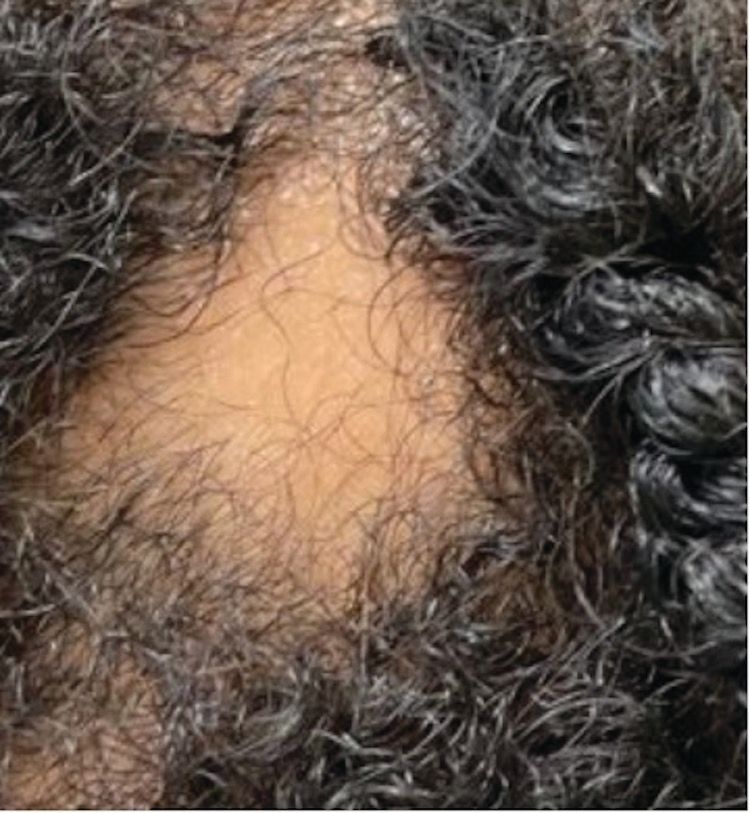
Alopecia areata (AA) is a chronic, immune-mediated disorder that presents as sudden-onset, nonscarring hair loss frequently in childhood or adolescence (Figure 1). Hair loss in AA is typically focal or patchy but can progress to alopecia totalis (AT; loss of all scalp hair) or alopecia universalis (AU; loss of all hair). There is an association between AA and other autoimmune diseases, namely vitiligo and thyroid disease.9 Interestingly, AA may exhibit seasonal variation, worsening during colder months and improving in warmer temperatures.10
AA progression is difficult to predict. Childhood onset may be a poor prognostic indicator; however, prognosis likely varies by severity, disease duration, and disease activity.11 Patients with highly active disease may be more likely to progress to AU or AT.11
AA can profoundly impact social and emotional well-being. Many children experience social phobia, anxiety, and depression, and they are more susceptible to bullying, social isolation, and stigmatization by peers.12-16 Even when patients experience hair regrowth, the unpredictability of losing it again impacts their psychological well-being.1 Thus, AA is far from a cosmetic disorder, and treatment of psychiatric comorbidities is critical.
First-line treatment for children with limited disease includes topical or injected corticosteroids. Topical immunotherapy with a contact allergen (eg, diphenylcyclopropenone or squaric acid dibutyl ester) is an option for focal or extensive disease. This involves application of an irritating solution to a small area of skin to sensitize the patient, followed by weekly application directly to areas of hair loss. Severe dermatitis is a potential adverse effect.
Recently, there has been excitement about Janus kinase (JAK) inhibitors for severe or refractory AA. However, JAK inhibitors carry a risk of severe adverse effects, including infection and possibly malignancy.14 Dupilumab, an IL-4 receptor blocker (approved for atopic dermatitis in children older than 6 years), may promote hair regrowth in patients with comorbid atopic dermatitis.17
Telogen effluvium
Telogen effluvium (TE) is one of the most common causes of hair loss. Hair has 3 distinct growth phases: anagen, the growth phase (marked by high mitotic rates); catagen, the regression phase; and telogen, the resting phase. TE results from abnormal transition of hairs from the anagen phase to the telogen phase, resulting in diffuse hair shedding. TE can be precipitated by medications, illness, surgery, metabolic disturbances, nutritional deficiencies, severe emotional distress, immunizations, and the postpartum state (Table 2).4 However, no trigger is identified in up to 30% of cases.4
Acute TE involves active hair shedding, it can last for up to 6 months, and it occurs in patients of all ages. Hair loss is typically observed 3 months after the inciting trigger. Chronic TE lasts for greater than 6 months and is more common in middle-aged to older women than children or adolescents. Many medications are linked to TE (Table 2). Interestingly, there may be seasonal variation, with increased incidence of TE in the summer months due to greater UV exposure.18
Physical examination will reveal reduced hair density, which is typically diffuse rather than patchy. Progression to complete loss of hair is uncommon.19 Some patients may exhibit patterned hair loss in the bitemporal, frontal, or vertex areas.19 Patients may also endorse tenderness, pain, burning, itching, or stinging of their scalp.
Acute TE resolves when the inciting factor is eliminated and is followed by hair regrowth over a 6- to 12-month period. Therefore, identifying and addressing the underlying insult is the most important step in treatment. Depending on the inciting factor, treatment could involve dietary supplementation, discontinuation of the suspected drug, addressing an underlying illness, or addressing concomitant hair or scalp disorders. As with any form of hair loss, psychological support is critical. It may be reassuring for patients to know that complete hair loss is not anticipated. Educating patients and families that noticeable hair regrowth may take up to 1 year can help set expectations. There is insufficient evidence to support the efficacy of topical medications, such as minoxidil, for TE.
Trichotillomania
Trichotillomania is a compulsive disorder of recurrent hair pulling that causes hair loss, which is more common in adolescent girls, and frequently affects the scalp, eyebrows, eyelashes, and pubic area.20 Trichotillomania can be classified as automatic (habitual) or focused (compulsive).20 In the automatic subtype, hair pulling is subconscious, whereas in the focused subtype, patients direct attention to the act of hair pulling itself in response to a stressor or urge.20 Triggers can include anger, school- or family-related stressors, self-image anxieties, or concurrent illness.20
Trichotillomania presents with patchy hair loss with irregular or geometric borders, disproportionately affecting the patient’s dominant side (Figure 2). Within a patch of hair loss, the hair shafts are varying lengths due to the temporal spacing of hair pulling and different breakage points. The integrity of the hair follicle is preserved; thus, a hair pull test will be negative.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) categorizes trichotillomania under obsessive-compulsive and related disorders. According to DSM-5 criteria, hair pulling must be recurrent, result in hair loss, and cause significant distress or functional impairment despite multiple attempts to decrease or stop the behavior. Trichotillomania is highly comorbid with other psychiatric disorders, particularly obsessive-compulsive disorder (OCD), mood disorders, and generalized anxiety disorder.21 Patients may be in denial about their behavior, and teens may be more likely to experience a chronic, relapsing course.20
Patients with trichotillomania should be referred for psychiatric evaluation and support. Habit reversal therapy, a cognitive behavioral therapy focused on awareness; stimulus control; relaxation; and social support are the first-line treatments.22 There is insufficient evidence to support the efficacy of pharmacologic interventions, including selective serotonin reuptake inhibitors, tricyclic antidepressants, naltrexone, and antipsychotics.23
Diagnosis and evaluation
History
Relevant personal or family history, including a history of AGA, metabolic syndrome, atopy, endocrinopathies, hyperthyroidism or hypothyroidism, and autoimmune conditions or psychiatric conditions, can help guide diagnosis and treatment. For example, most children with AGA will have a family history of AGA, and patients with trichotillomania are more likely to have a comorbid psychiatric condition.3,20
It is important to collect a dietary history to assess nutritional status. Deficiencies in iron, zinc, biotin, and vitamin D are implicated in TE. Severe caloric or protein malnutrition can also precipitate hair loss. Additionally, a recent history of a new medication, significant illness, weight loss, emotional or psychological trauma, infection, or toxin exposure may support a diagnosis of TE, particularly if the occurrence is within the typical time frame of 3 to 4 months prior to the onset of hair loss.
Asking patients about their menstrual period can help assess conditions related to a hyperandrogenic state (ie, PCOS) or other endocrinological disorders, such as hypothalamic amenorrhea.
Physical examination
Hair and scalp
Physical examination should focus on the scalp and hair. Is the hair loss diffuse or focal? Are the hair shafts uniform or varying lengths? Are the follicular ostia preserved? Do you see scales, scarring, or pustules? Are the eyebrows or eyelashes affected?
For example, a classic male pattern hair loss in AGA presents as thinning of the hair at the vertex and bitemporal regions, whereas female pattern hair loss in AGA presents with thinning and widening of the middle part.3 On the other hand, smooth and discrete areas of nonscarring hair loss are typical of AA. When observed, exclamation point hairs (short, broken hairs with narrowing of the proximal end) are pathognomonic for AA. The presence of scales, pustules, and scarring (loss of follicular ostia) may suggest an inflammatory condition (eg, seborrheic dermatitis, psoriasis) or an infection (eg, tinea). A strange or irregular hair loss pattern that affects the patient’s dominant side with varying hair shaft lengths indicates trichotillomania.
Eyebrows and eyelashes
Careful examination of the eyebrows and eyelashes may reveal the following24:
· AA: Patchy loss of eyebrow hair or eyelashes can be the sole manifestation of AA or indicate progression of AA to AT or AU. Hairs are uniform in length, which helps distinguish AA from trichotillomania.
· TE: Diffuse loss of eyebrow hair
· Seborrheic dermatitis: Erythema or scaling of the eyebrows
· Hypothyroidism: Loss of the lateral one-third of the eyebrow (Hertoghe sign), also observed in atopic dermatitis (due to rubbing/scratching)
· Trichotillomania: Irregular patches of eyebrow hair or eyelashes of varying lengths
Nails
Pitting, onycholysis, brittle nails, or thickening may indicate an inflammatory condition such as psoriasis. The presence of transverse grooves (Beau lines) may signify a recent illness, supporting a diagnosis of TE. Other nutritional deficiencies present with distinct nail findings; for example, iron deficiency can cause koilonychia (nail spooning).
Other
Clinical signs of hyperandrogenism, such as acne, seborrhea, menstrual irregularities, and hirsutism, can support a diagnosis of AGA in the appropriate clinical context. Short stature, dysmorphic facial features, vision or hearing impairment, discordant Tanner staging, and signs of virilization may indicate an underlying genetic, metabolic, or endocrine disorder and should prompt additional diagnostic evaluation and possibly subspecialist referral (eg, endocrinology, genetics).
Lymph node palpation (posterior cervical, suboccipital) can be helpful in evaluating for an inflammatory or infectious (bacterial, fungal) etiology.
Diagnostic tests
Hair pull test
A hair pull test is performed by grasping 50 to 60 hairs proximal to the scalp between the thumb, index, and long finger.25 The hairs are then pulled slowly along the hair shaft, avoiding a fast or forceful tug. Removal of more than 2 hairs during this test is considered positive.25 This test can help confirm active hair loss. A positive test in a focal area may indicate AA, whereas a positive test in multiple areas may suggest TE. The test will be negative in AGA and trichotillomania. The hair pull test can also be used to monitor the active edge of AA.
Laboratory evaluation
A complete blood count and complete metabolic panel should be considered for all adolescents with hair loss to reveal any treatable underlying illness, metabolic abnormality, or nutritional deficiency. If there is high clinical suspicion for supplement or environmental exposure–related metal toxicity (eg, lead, cadmium, thallium, mercury, arsenic, selenium, vitamin A, colchicine), systemic levels should be evaluated.26 Although serum androgen levels are often within a developmentally appropriate range, if a child has other signs of virilization, a complete hormonal workup can be considered.
Thyroid function screening should be considered in patients with AA, particularly in children with Down syndrome, a personal history of atopy, a family history of thyroid disease, or clinical findings suggestive of thyroid dysfunction.9
Fungal tests
If an infectious etiology is suspected, a swab and culture of an affected scalp area should be performed. An in-office skin potassium hydroxide (KOH) examination of a skin scraping can rapidly identify fungal hyphae if present. However, a fungal culture is more specific and recommended, even if KOH examination is nondiagnostic. Of note, sampling an intensely inflammatory lesion (eg, a kerion) can yield false-negative cultures.4
Trichoscopy
Trichoscopy has emerged as the gold standard for diagnosing hair loss and is generally preferred over biopsy. Referral to a dermatologist who specializes in hair loss or who is comfortable with trichoscopy can be helpful for clinically challenging cases. Trichoscopy findings are beyond the scope of this review.
Biopsy
A biopsy may be necessary in cases where the diagnosis is uncertain, although it is generally reserved as a last resort, particularly in children.
Psychiatric evaluation
Hair loss in children and adolescents often impacts self-esteem and social interactions and can lead to clinically significant depression, anxiety, or emotional dysregulation.12,16 Any adolescent who presents with hair loss should undergo psychiatric evaluation. Certain conditions, such as TE, may be a manifestation of an underlying psychiatric disorder (eg, OCD, anxiety, mood or impulse control disorders, substance dependency, bulimia nervosa) or emotional distress. In such cases, addressing the underlying stressor or disorder is integral to treating the associated hair loss.
Supportive management
Some patients may wish to camouflage their hair loss; wigs can be a helpful supportive measure and may positively impact patient quality of life.27 Psychological support, including behavioral therapy, support groups, and treatment of comorbid depression, anxiety, or other psychiatric conditions, should also be offered and may be the primary treatment modality for certain hair loss disorders, such as trichotillomania.
Although hair styling practices are not a focus of this review, these can contribute to hair loss (ie, traction alopecia). Patients should be counseled in a culturally sensitive manner to limit high-risk hairstyles, such as tight buns, ponytails, braids, cornrows, dreadlocks, and application of weaves, braids, or hair extensions to relaxed hair.4 Painful hairstyles that lead to the development of pustules or crusting should be avoided.
Conclusion
Hair loss in adolescents encompasses a broad range of etiologies. A thorough history and physical examination is essential to narrow the differentials and facilitate a tailored diagnostic evaluation. Treatment includes pharmacologic, behavioral, and supportive measures. LDOM, JAK inhibitors, and dupilumab are promising new therapies and require further evaluation of safety and efficacy in pediatric patients. Because alopecia can be extremely distressing and may present with a variety of comorbid psychiatric conditions, mental health evaluation and support is a crucial component of management.
For more from the September issue of Contemporary Pediatrics®, click here.
References:
1. de Vere Hunt I, McNiven A, McPherson T. A qualitative exploration of the experiences of adolescents with alopecia areata and their messages for healthcare professionals. Br J Dermatol. 2021;184(3):557-559. doi:10.1111/bjd.19598
2. Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163(2):378-385. doi:10.1111/j.1365-2133.2010.09777.x
3. Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85(5):1267-1273. doi:10.1016/j.jaad.2019.08.018
4. Xu L, Liu KX, Senna MM. A practical approach to the diagnosis and management of hair loss in children and adolescents. Front Med (Lausanne). 2017;4:112. doi:10.3389/fmed.2017.00112
5. Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97(2):166-172. doi:10.1016/j.abd.2021.06.006
6. Georgala S, Befon A, Maniatopoulou E, Georgala C. Topical use of minoxidil in children and systemic side effects. Dermatology. 2007;214(1):101-102. doi:10.1159/000096924
7. de Nicolas-Ruanes B, Moreno-Arrones OM, Saceda-Corralo D, et al. Low-dose oral minoxidil for treatment of androgenetic alopecia and telogen effluvium in a pediatric population: a descriptive study. J Am Acad Dermatol. 2022;87(3):700-702. doi:10.1016/j.jaad.2022.04.030
8. Olamiju B, Craiglow BG. Combination oral minoxidil and spironolactone for the treatment of androgenetic alopecia in adolescent girls. J Am Acad Dermatol. 2021;84(6):1689-1691. doi:10.1016/j.jaad.2020.10.097
9. Patel D, Li P, Bauer AJ, Castelo-Soccio L. Screening guidelines for thyroid function in children with alopecia areata. JAMA Dermatol. 2017;153(12):1307-1310. doi:10.1001/jamadermatol.2017.3694
10. Putterman E, Castelo-Soccio L. Seasonal patterns in alopecia areata, totalis, and universalis. J Am Acad Dermatol. 2018;79(5):974-975. doi:10.1016/j.jaad.2018.06.029
11. Jang YH, Eun DH, Kim DW. Long-term prognosis of alopecia areata in children and adolescents. Ann Dermatol. 2019;31(2):231-234. doi:10.5021/ad.2019.31.2.231
12. Altunisik N, Ucuz I, Turkmen D. Psychiatric basics of alopecia areata in pediatric patients: evaluation of emotion dysregulation, somatization, depression, and anxiety levels. J Cosmet Dermatol. 2022;21(2):770-775. doi:10.1111/jocd.14122
13. Vélez-Muñiz RDC, Peralta-Pedrero ML, Jurado-Santa Cruz F, Morales-Sánchez MA. Psychological profile and quality of life of patients with alopecia areata. Skin Appendage Disord. 2019;5(5):293-298. doi:10.1159/000497166
14. Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76(1):29-32. doi:10.1016/j.jaad.2016.09.006
15. Macey J, Kitchen H, Aldhouse NVJ, et al. A qualitative interview study to explore adolescents' experience of alopecia areata and the content validity of sign/symptom patient-reported outcome measures. Br J Dermatol. 2022;186(5):849-860. doi:10.1111/bjd.20904
16. Creadore A, Manjaly P, Li SJ, et al. Evaluation of stigma toward individuals with alopecia. JAMA Dermatol. 2021;157(4):392-398. doi:10.1001/jamadermatol.2020.5732
17. McKenzie PL, Castelo-Soccio L. Dupilumab therapy for alopecia areata in pediatric patients with concomitant atopic dermatitis. J Am Acad Dermatol. 2021;84(6):1691-1694. doi:10.1016/j.jaad.2021.01.046
18. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen effluvium: a review of the literature. Cureus. 2020;12(5):e8320. doi:10.7759/cureus.8320
19. Trüeb RM. Systematic approach to hair loss in women. J Dtsch Dermatol Ges. 2010;8(4):284-298. doi:10.1111/j.1610-0387.2010.07261.x
20. Mosca M, Martin K, Hadeler E, Hong J, Brownstone N, Koo J. Review of the diagnosis and management of pediatric psychodermatologic conditions: part I. Pediatr Dermatol. 2022;39(1):17-21. doi:10.1111/pde.14888
21. Gerstenblith TA, Jaramillo-Huff A, Ruutiainen T, et al. Trichotillomania comorbidity in a sample enriched for familial obsessive-compulsive disorder. Compr Psychiatry. 2019;94:152123. doi:10.1016/j.comppsych.2019.152123
22. Farhat LC, Olfson E, Nasir M, et al. Pharmacological and behavioral treatment for trichotillomania: an updated systematic review with meta-analysis. Depress Anxiety. 2020;37(8):715-727. doi:10.1002/da.23028
23. Hoffman J, Williams T, Rothbart R, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2021;9(9):Cd007662. doi:10.1002/14651858.CD007662.pub3
24. Kumar A, Karthikeyan K. Madarosis: a marker of many maladies. Int J Trichology. 2012;4(1):3-18. doi:10.4103/0974-7753.96079
25. McDonald KA, Shelley AJ, Colantonio S, Beecker J. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76(3):472-477. doi:10.1016/j.jaad.2016.10.002
26. Yu V, Juhász M, Chiang A, Atanaskova Mesinkovska N. Alopecia and associated toxic agents: a systematic review. Skin Appendage Disord. 2018;4(4):245-260. doi:10.1159/000485749
27. Inui S, Inoue T, Itami S. Psychosocial impact of wigs or hairpieces on perceived quality of life level in female patients with alopecia areata. J Dermatol. 2013;40(3):225-226. doi:10.1111/1346-8138.12040

Newsletter
Access practical, evidence-based guidance to support better care for our youngest patients. Join our email list for the latest clinical updates.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.