Dermatology
Latest News
Latest Videos
Shorts
Podcasts
CME Content
More News
Sub-analysis of ADORING trials shows VTAMA cream improved skin clearance, symptoms, and patient-reported outcomes in children aged 2-17.
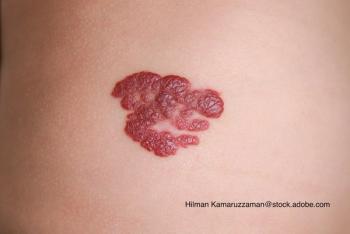
Can you guess the diagnosis?

A pair of phase 3 trials showed that AbbVie's daily upadacitinib 15 mg led to a 50% reduction in T-VASI 50 and a 75% reduction in F-VASI 75 at 48 weeks among adolescents 12 years and older.

New one-year data showed that baricitinib continued scalp, eyebrow, and eyelash regrowth in adolescents with severe alopecia areata.

A single injection once per 8 weeks vs once per 4 weeks demonstrated similar levles of skin clearance, for AD patients 12 years and older, according to a Fall Clinical announcement.

New patient- and caregiver-reported outcomes across 3 phase 3 trials demonstrated improved itch and reduced impact of AD on sleep in patients 2 years and older.

Indication expansions include hidradenitis suppurativa for patients 12 years and up, as well as for patients with uveitis aged 2 years and older.

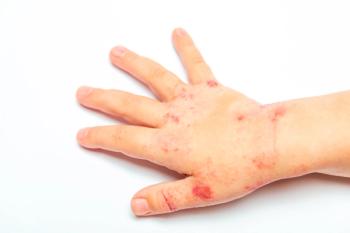
Can you guess the diagnosis?

The once-daily, steroid-free cream can be used anywhere on the body for treatment and for any duration, according to Arcutis.

Watch our FDA pipeline news recap for the month of September 2025 as well as a preview of a key PDUFA date in October.

Ingrid Polcari, MD, highlights concerns with teen skin care trends and emphasizes simple, age-appropriate routines tailored to patient needs.

Ingrid Polcari, MD, discusses acne severity, treatment options, and the importance of persistence and open discussion in pediatric care.

From another potential treatment for pediatric atopic dermatitis, to STI therapy for uncomplicated urogenital gonorrhea, take a look ahead at key PDUFA dates in Q4.

FDA approves guselkumab as the first IL-23 inhibitor for children 6 years or older with plaque psoriasis or psoriatic arthritis.
Rezpegaldesleukin improved disease severity and patient-reported outcomes in a phase 2b atopic dermatitis trial with sustained benefit.

The FDA has approved an additional indication for Incyte's ruxolitinib cream to include pediatric patients.
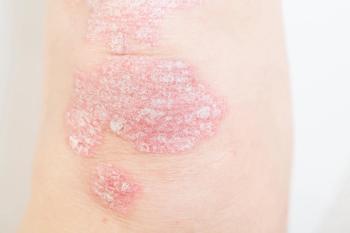
New phase 3 data show icotrokinra maintained high PASI90 responses in pediatric and adult patients at 1 year.

The updated label is based on real-world data collected since B-VEC was launched in the United States, following its approval in May 2023.

Arcutis has submitted an sNDA to the FDA for roflumilast cream 0.3% for plaque psoriasis in children aged 2 to 5 years.

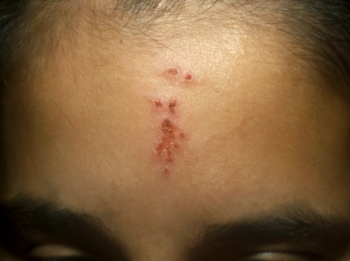
Can you guess the diagnosis?

The baby had mild respiratory distress after delivery and briefly required supplemental blow-by oxygen during transitioning.

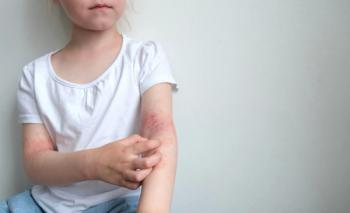
Can you guess the diagnosis?

In this paper, we provide several cases of human bite injuries, juxtaposed with cases of bite injuries from common animals.

Despite their frequency, it can be challenging to distinguish between types of bites. Can you identify the source of this bite mark?

Achieving the primary end point, 44.6% and 54.3% of patients treated with upadacitinib (15 mg and 30 mg) reached 80% or more scalp hair coverage.