Atopic Dermatitis
Latest News
Latest Videos

Shorts
CME Content
More News

Panelists discuss how calcineurin inhibitors can be safely and effectively used in children with proper education and clinical oversight.

A single injection once per 8 weeks vs once per 4 weeks demonstrated similar levles of skin clearance, for AD patients 12 years and older, according to a Fall Clinical announcement.

New patient- and caregiver-reported outcomes across 3 phase 3 trials demonstrated improved itch and reduced impact of AD on sleep in patients 2 years and older.

Panelists discuss how corticosteroids remain essential for controlling flares but must be used thoughtfully with education and reassurance for families.

Panelists discuss how individualized, consistent topical care helps build trust, promotes adherence, and prevents disease flare-ups in children with AD.

Panelists discuss how treatment goals should center on the child’s comfort, sleep quality, and emotional well-being, not just visible skin improvement.

Panelists discuss how atopic dermatitis affects not only the child’s comfort and confidence but also family well-being and emotional health.

A pair of roflumilast clinical trial investigators react to the FDA approval of the 0.05% formulation to treat atopic dermatitis in children aged 2 to 5 years.

The once-daily, steroid-free cream can be used anywhere on the body for treatment and for any duration, according to Arcutis.

From another potential treatment for pediatric atopic dermatitis, to STI therapy for uncomplicated urogenital gonorrhea, take a look ahead at key PDUFA dates in Q4.
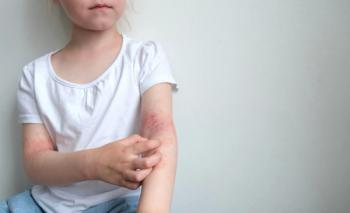
Rezpegaldesleukin improved disease severity and patient-reported outcomes in a phase 2b atopic dermatitis trial with sustained benefit.

The FDA has approved an additional indication for Incyte's ruxolitinib cream to include pediatric patients.
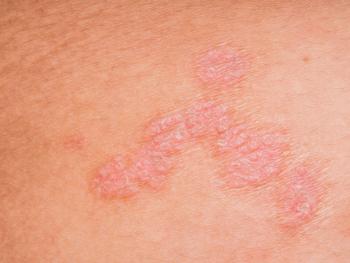
The study enrolled infants not selected for risk, a population that has been seldom studied with regard to emollient intervention.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Study links early childhood eczema timing to risk of allergies like food allergy, asthma, and rhinitis, with five distinct disease phenotypes identified.

John Browning, MD, highlights the importance of fragrance-free, steroid-free creams in managing atopic dermatitis safely and effectively in children.

Roflumilast cream 0.05% is being studied for mild to moderate AD in infants aged 3 months to under 2 years in a new phase 2 trial.

Lebrikizumab demonstrated efficacy and safety in patients with skin of color and moderate to severe atopic dermatitis in the ADmirable trial. Trial investigator Andrew Alexis, MD, MPH, reacts.

The FDA has extended the review of ruxolitinib for atopic dermatitis in children 2 to 11 years to September 2025 to evaluate new CMC data.

John Browning, MD, highlights why pediatricians should feel comfortable prescribing recent nonsteroidal medications and what questions to ask patients in the office.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The phase 2 trial will be 4 weeks and evaluate investigational, once-daily roflumilast cream 0.05% in infants 3 months to less than 2 years of age.
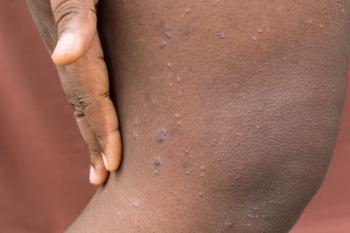
Results at 24 weeks revealed that 76% of individuals achieved a 75% or more improvement in overall disease severity (EASI-75), trial's primary endpoint.

Mona Shahriari, MD, discusses how RAD 2025 can equip providers with tools to improve atopic dermatitis outcomes.

View our Q1 2025 recap of standout pediatric news from FDA regulatory updates, clinical trial results, and expert commentary.