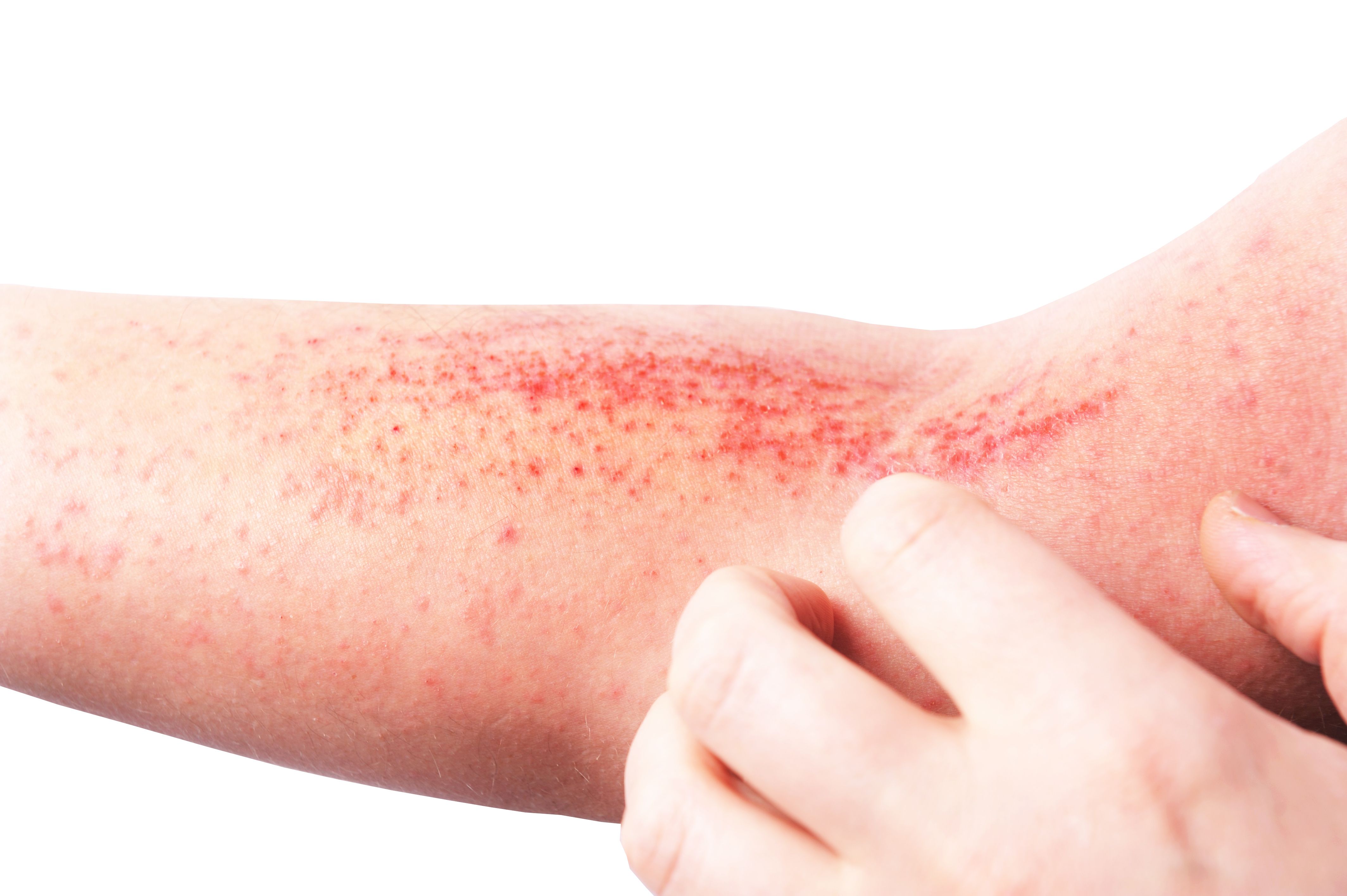
Atopic Dermatitis
Latest News
Latest Videos

More News

According to new study data presented at the 2024 Pediatric Academic Societies Meeting, dupilumab (dupixent; Sanofi and Regeneron) demonstrated positive safety and efficacy results for up to 1 year in infants and preschool-age children with atopic dermatitis.

The resubmission was announced in a first quarter, 2024 earnings news release from Lilly, which expects "regulatory action in the second half of 2024."

A decision from the federal agency is expected in the fourth quarter of 2024.
Data from a pair of identical, phase 3, double-blind, randomized, and vehicle-controlled trials were presented at the 2024 American Academy of Dermatology (AAD) Annual Meeting in San Diego, California.

Lawrence Eichenfield, MD, highlights the sNDA submission of tapinarof cream, 1% for the topical treatment of atopic dermatitis in children aged 2 years and older.

The sNDA submission follows additional positive topline data that was presented in Janurary 2024, highlighting an open-label, long-term extension study evaluating tapinarof cream, 1%.

In this Contemporary Pediatrics Q+A interview, Weily Soong, MD, breaks down FDA-approved tralokinumab-ldrm for patients aged 12 to 17 years with moderate-to-severe atopic dermatitis.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
According to new pooled individual patient responses, roflumilast cream 0.15% treatment led nearly 92% of individuals to achieve a measurable improvement in the Eczema Area and Severity Index.

New interim data from the on-going, long-term extension ADORING 3 study and from an integrated analysis of the entire ADORING development program revealed efficacy and safety using tapinarof cream 1% continued beyond 8 weeks of treatment.

Lawrence Eichenfield, MD, discusses topical steroid, non-steroidal, and potentially new atopic dermatitis treatments for the pediatric population.

The FDA has accepted a supplemental New Drug Application (sNDA) for roflumilast cream 0.15% for the treatment of atopic dermatitis (AD) in children aged 6 years and up.

Of patients in the atopic dermatitis (AD) cohort, 36.6% developed at least 1 comorbidity amid follow-up compared to 28.5% in the non-AD reference cohort, investigators of a Sweden, nationwide, population-based cohort study found.

Of the patients that responded to lebrikizumab at week 16 in the phase 3 trials ADvocate 1 and ADvocate 2, 84% achieved a clinically meaningful response in at least 1 domain of the disease (mild signs, symptoms, or quality of life impact) at 52 weeks.

A rapid reduction in pruritis as early as 24 hours after first application was announced as new positive data from a pair of identical, phase 3 studies of tapinarof cream 1% in children as young as 2 years and adults with atopic dermatitis (AD).

Positive topline results from a pair of identical phase 3 trials support the submission of a supplemental New Drug Application to the FDA for Arcutis Biotherapeutics’ roflumilast cream 0.15%, a once-daily topical to treat mild to moderate atopic dermatitis (AD) in children 6 years or older.
Based on recent positive phase 3 results, Arcutis Biotherapeutics,. intends to submit a supplemental New Drug Application with the FDA for roflumilast cream 0.05% to treat mild to moderate atopic dermatitis in children aged 2 to 5 years.

In her presentation ‘Treatment of Eczema in Pediatric Patients Under 12 Years,’ Dr. Garza-Mayers spoke on considerations for atopic dermatitis management.
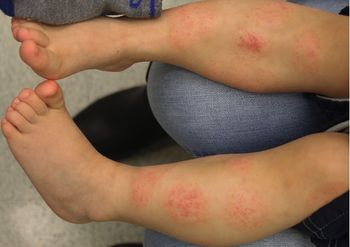
A healthy 2-year-old boy with atopic dermatitis developed intermittent flares of eczematous patches on his legs with dramatic sparing below the sock line following meals. What is the diagnosis?

The once-daily topical cream for the treatment of atopic dermatitis (AD) has demonstrated positive topline results in 2 phase 3 studies in adults and children aged 2 years and up. According to Dermavant, a supplemental new drug application (sNDA) filed with the FDA is anticipated in Q1 of 2024.
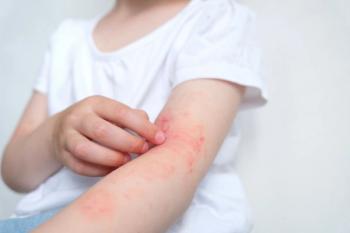
In identical phase 3 trials, roflumilast demonstrated topline positive results, reducing itch in children and adults with mild to moderate AD.
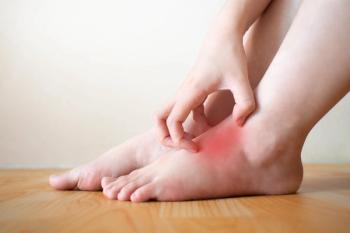
In a phase 3 clinical trial, dupilumab met both its primary and key secondary endpoints, demonstrating efficacy as a potential treatment for atopic hand and foot dermatitis.

At the 44th National Association of Pediatric Nurse Practitioners Conference, methods of treating atopic dermatitis were discussed.

A recent study indicated that lebrikizumab treatment for 16 weeks was effective for adolescents and adults with moderate-to-severe atopic dermatitis.
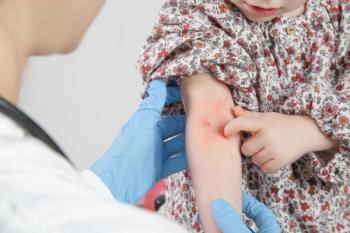
Results from a recent phase 3 clinical trial demonstrated topline safety and efficacy results for tapinarof cream, 1% (VTAMA; Dermavant) as a potential treatment for atopic dermatitis in patients 2 years and older.