Rheumatology
Latest News
Latest Videos
CME Content
More News

The combination therapy yielded boosted treatment benefits of navepegritide, including improvement in mean ACH Z-score and mean annualized growth.

With the new drug application accepted with priority review, the FDA has assigned a date of November 30, 2025, to complete its review for potential approval.

The expanded indication follows original approval in August 2018 for use in previously-treated patients 12 years and older.

Delandistrogene moxeparvovec-rokl (ELEVIDYS) showed significant motor function improvements in 8- to 9-year-old Duchenne muscular dystrophy patients in part 2 of the phase 3 EMBARK study.

Stealth BioTherapeutics' CEO stated, "We hope to gain more information on the revised action date in the coming days."

The human FcRn-blocking monoclonal antibody is indicated for gMG patients aged 12 years and older.

Submission is based on 3 clinical trials, including data from the ApproaCH Trial among children with achondroplasia.

Fitusiran is administered subcutaneously starting once every 2 months for patients aged 12 years and older with hemophilia A or hemophilia B, with or without factor VIII or IX inhibitors.

Diazoxide choline has been granted breakthrough, fast track, and orphan drug designations in the United States.

In this Q+A interview, Craig McDonald, MD, discusses the interim, 90-day data from the phase 1/2 INSPIRE DUCHENNE trial, evaluating SGT-003, a gene therapy candidate for DMD.
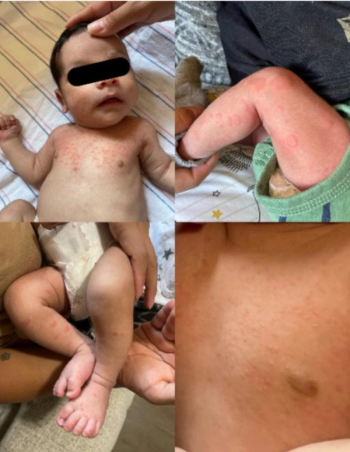
Can you diagnose this patient in our most recent puzzler poll?

As a biosimilar to Actemra, tocilizumab-anoh in both IV and SC formulation is approved to treat rheumatoid arthritis, pJIA, sJIA, COVID-19, and giant cell arteritis.
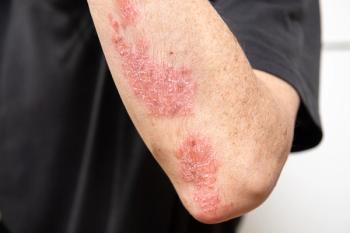
The submission to treat PsO is for children aged 6 years and up while the jPsA submission is to treat children aged 5 years and older.

101-PGC-005 is Type IA prodrug of dexamethasone that targets CD206+ macrophages.

The approval makes methotrexate, currently, the only only oral liquid methotrexate on the market approved for both adult and pediatric indications.

Approval is based on a study in 116 adult and pediatric male patients with either severe hemophilia A or severe hemophilia B, both without inhibitors.

Infigratinib has also received Orphan Drug Designation, Fast Track Designation, and Rare Pediatric Disease Designation from the FDA.
An 11-year-old boy with a history of asthma and allergic rhinitis presented to the emergency department (ED) with worsening fatigue, minimal responsivity to external stimuli, and diffuse muscle weakness for 2 months.

Current treatment is primarily supportive care consisting of increased fluid intake to dilute oxalate in the urine, along with pyridoxine, or vitamin B6, to reduce oxalate production.

Jon Matthew Farber, MD, offers quick thoughts on a recent study regarding bone health and activity among adolescents withJIA.

An intentional launch date for ustekinumab-ttwe in the United States is set for sometime in February 2025.

pJIA can lead to an increased risk of permanent joint damage as well as delayed growth and development.
Additionally, a new weight-based oral solution, RINVOQ LQ, is now available as an option for the pediatric populations.

Psoriatic arthritis and spondyloarthritis occurred earlier in children and adolescents with IBD than in the matched references without IBD.

Tocilizumab-bavi (Tofidence; Biogen) demonstrated a biosimilarity to tocilizumab (Actemra; Genentech) based on multifaceted clinical and non-clinical data for polyarticular juvenile idiopathic arthritis (PJIA) and systemic juvenile idiopathic arthritis (sJIA) in children aged 2 years and up. This makes it the first tocilizumab biosimilar to be FDA-approved in the United States, according to Biogen.