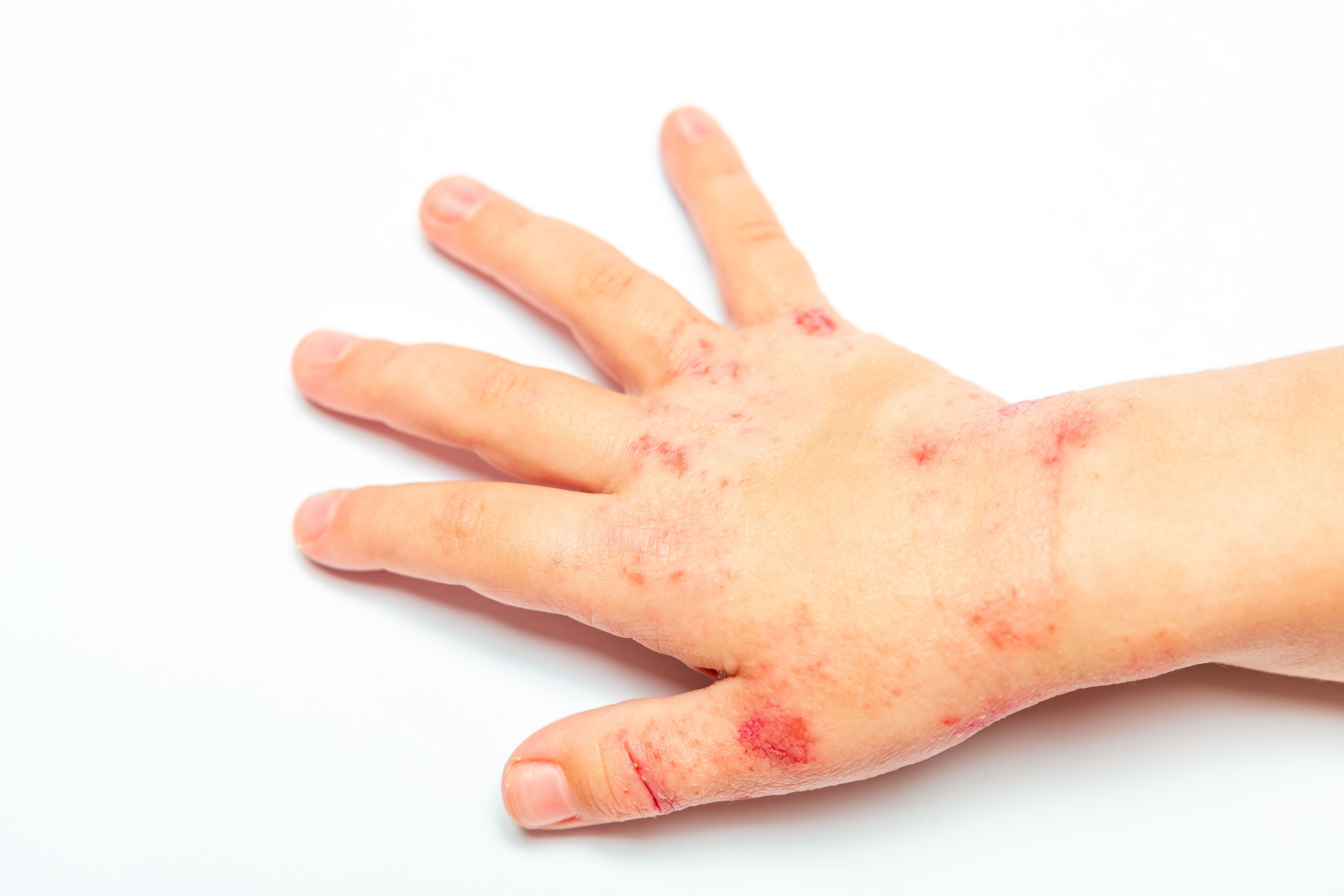
Atopic Dermatitis
Latest News
Latest Videos

More News

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Lawrence Eichenfield, MD, talks tapinarof cream, 1%, nemolizumab FDA approvals for atopic dermatitis
"Tapinarof comes in with that mixture of the short-term studies and longer-term studies intermittently, giving us a nice, effective alternative non-steroid for eczema across the ages."

From new topical dermatology treatments for atopic dermatitis to the first nasal spray to treat type 1 allergic reactions, these are our top FDA approvals of 2024.

Arcutis seeks FDA approval for roflumilast cream 0.05% (ZORVYE) to treat mild to moderate atopic dermatitis in children 2 to 5 years.

According to Galderma, approval is based on positive results from the phase 3 ARCADIA clinical trial program.

"I really hope this becomes a first-line agent that people are comfortable prescribing whether they are in dermatology, pediatric dermatology, or primary care."
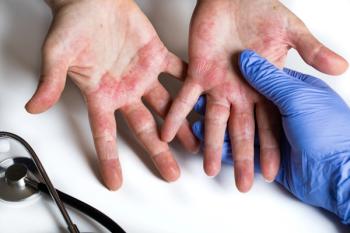
In the study through 76 weeks, no new risks were observed with once-daily upadacitinib (15 mg or 30 mg).
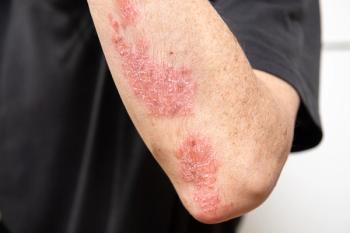
The submission to treat PsO is for children aged 6 years and up while the jPsA submission is to treat children aged 5 years and older.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The federal agency has not raised any concerns regarding safety and efficacy of tapinarof cream, 1%.

In all, 51.9% of patients entered with or achieved complete disease clearance at least once during the 48-week study.

A recent study presented at the American Academy of Pediatrics 2024 National Conference & Exhibition, sheds light on the connection between skin conditions and sleep disturbances in infants and toddlers, highlighting itchy skin as a significant factor, even in the absence of atopic

Eric Simpson, MD, MCR, FAAD, lead author of a recently-published article highlighting roflumilast cream 0.15%, joined us for a Q+A interview.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

The indication is supported by trial data showing three-fourths of successfully treated patients maintained clear skin through 1 year of a lebrikizumab regimen.

Efficacy of roflumilast cream 0.05% improved over time among children aged 2 to 5 years with mild to moderate atopic dermatitis.

Details of newly FDA-approved roflumilast cream 0.15% for AD in pediatric patients, plus insight and commentary from Lawrence Eichenfield, MD.

The decision target action date was July 7, 2024. At this time, there is no indication from the federal agency to extend the date further.

Once-daily, steroid-free, topical roflumilast cream 0.15% has a PDUFA date of July 7, 2024.

What are the skin conditions in which itching becomes the dominant clinical symptom in children?
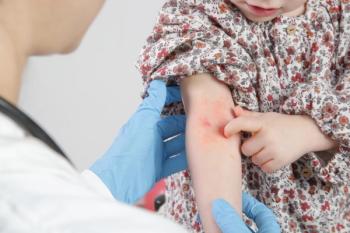
These data from investigators at Trinity College Dublin highlighted the immune response mechanisms visible in children with atopic dermatitis.

According to new study data presented at the 2024 Pediatric Academic Societies Meeting, dupilumab (dupixent; Sanofi and Regeneron) demonstrated positive safety and efficacy results for up to 1 year in infants and preschool-age children with atopic dermatitis.

The resubmission was announced in a first quarter, 2024 earnings news release from Lilly, which expects "regulatory action in the second half of 2024."

A decision from the federal agency is expected in the fourth quarter of 2024.
Data from a pair of identical, phase 3, double-blind, randomized, and vehicle-controlled trials were presented at the 2024 American Academy of Dermatology (AAD) Annual Meeting in San Diego, California.