The parents of a 3-month-old girl seek medical advice regarding recent seizures and a facial birthmark. What’s the diagnosis?

The parents of a 3-month-old girl seek medical advice regarding recent seizures and a facial birthmark. What’s the diagnosis?

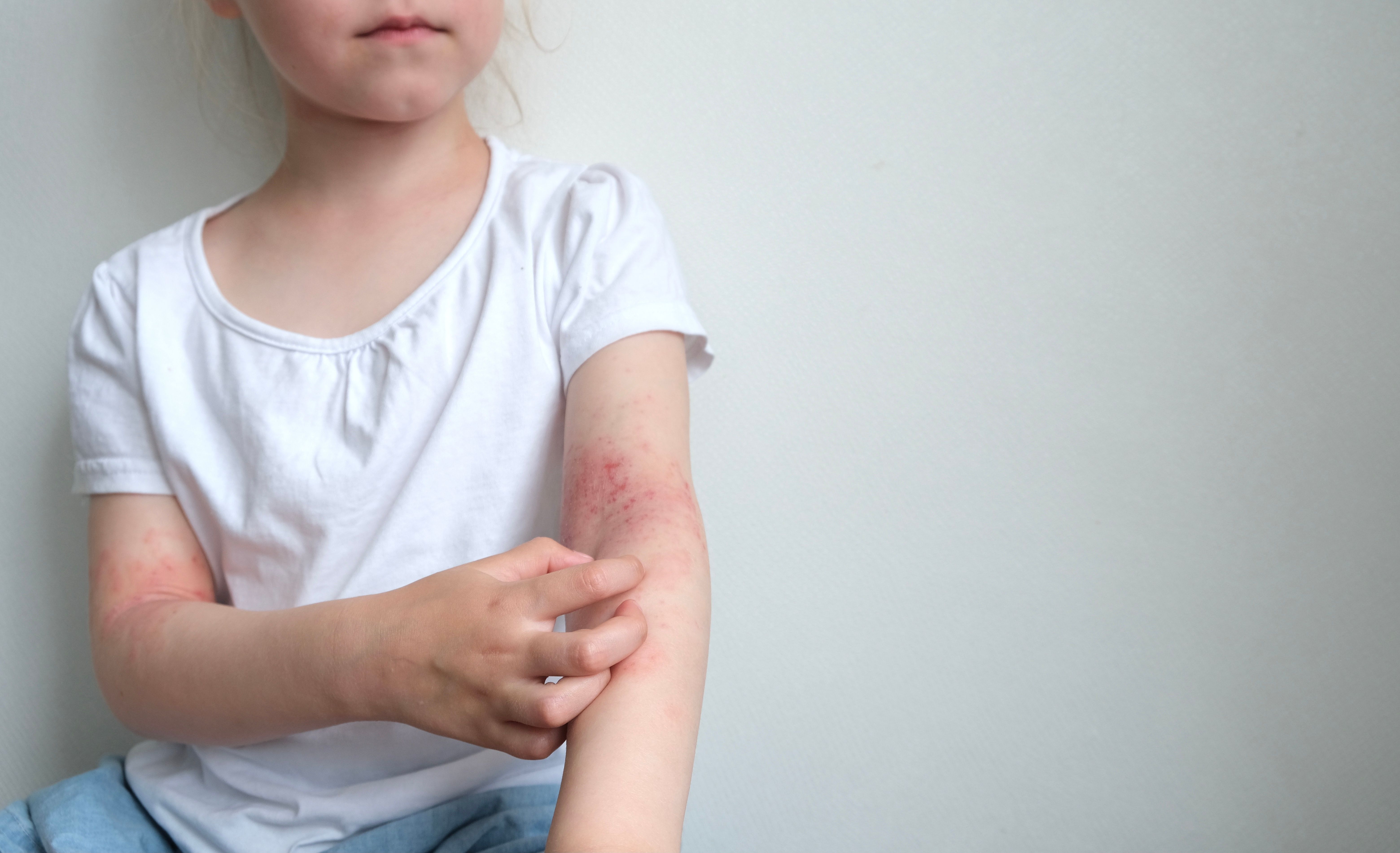
New research suggests the sleep quality of caregivers and patients with atopic dermatitis (inadequately-controlled with topical therapies) may improve with dupilumab treatment.
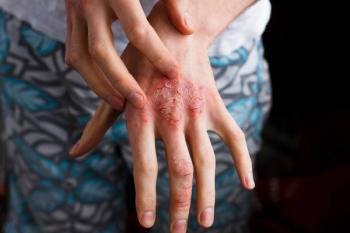
New 16-week data from the SPROUT trial show the PDE4 inhibitor may be highly efficacious in children and adolescents.
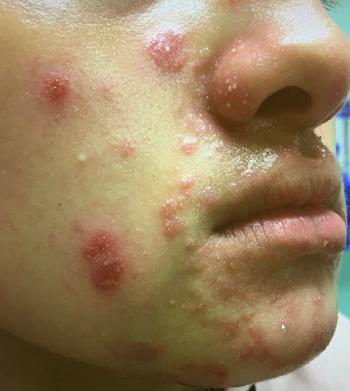
This case study analyzes variants of majocchi granuloma and potential treatment.

At the 2022 AAP National Conference & Exhibition, a presenter delves into diagnosis and management of common and uncommon skin rashes in newborns.
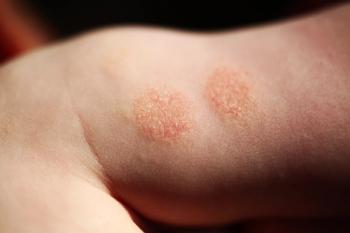
A review examined the ways in which costs can be reduced for patients with varying types of atopic dermatitis.
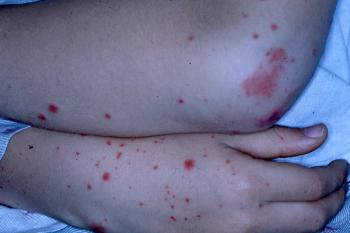
A previously healthy 11-year-old boy presents with purpuric macules and papules symmetrically distributed on his buttocks and upper and lower extremities. What’s the diagnosis?
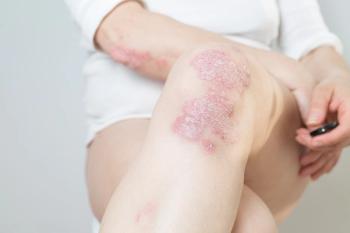
In 2 recent phase 3 trials, safety and efficacy was proven in the use of roflumilast cream 0.3% for the treatment of plaque psoriasis.
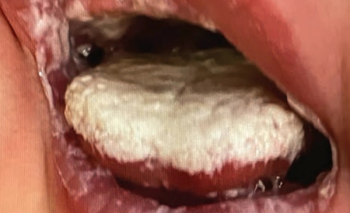
A 4-week-old boy presents with creamy white patches on his lips, right and left buccal mucosa, palate, and tongue.

Investigators have found that there are limited options for treating pediatric psoriasis.

Janssen Pharmaceutical Companies of Johnson & Johnson announced the approval of ustekinumab as a new treatment option for patients aged 6 years or older with active psoriatic arthritis.

Arcutis Biotherapeutics’ roflumilast cream will provide relief to patients aged 12 years or older diagnosed with plaque psoriasis.
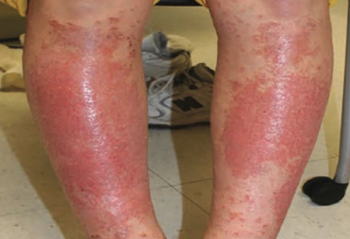
A healthy 14-year old boy was evaluated for an intensely pruritic shin rash that developed 2 weeks prior and had been treated with oral antibiotics for 10 days.

Stephen Stripling, MD, FAAP, discusses results published in JAMA Dermatology about the efficacy and safety of berdazimer gel 10.3% for the treatment of molluscum contagiosum.

Opzelura (ruxolitinib) cream is the first and only FDA-approved treatment for adult and pediatric patients 12 years of age or older with vitiligo.

A better understanding of this atopic diathesis and the atopic march often seen in pediatric patients is necessary to optimally treat and manage the patient population, ideally in a multidisciplinary setting.

Investigators suggest parents and clinicians should be mindful of children with suspected ACD with a history of exposure to jewelry, clothing, and belts with cobalt.

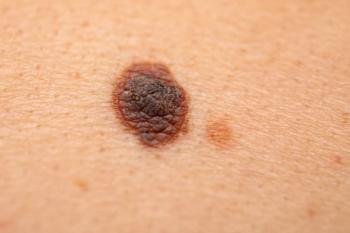
At an SPD 2022 47th Annual Meeting poster session, researchers look at gender and age differences in pediatric patients with melanoma.
At the SPD 2022 47th Annual Meeting, a poster session looks at the updated guidelines for rosacea, and whether they are inclusive of children.

At the SPD 2022 47th Annual Meeting, we chat with Albert Yan, MD, of Children's Hospital of Philadelphia on the latest trends in technology for pediatric dermatologists.

At the SPD 2022 47th Annual Meeting, a recap of don’t-miss sessions for Sunday, July 10.

At the SPD 2022 47th Annual Meeting, a very funny—and informative—discussion on the pros and cons of telehealth in dermatology.

At the SPD 2022 47th Annual Meeting in Indianapolis, a pediatric dermatologist shares his views on what we have learned From the COVID-19 pandemic.

At the SPD 2022 47th annual meeting, investigators share data on the treatment of ruxolitinib for this chronic autoimmune disease.

At the SPD 2022 47th Annual Meeting, a discussion of the latest therapeutics for acne.