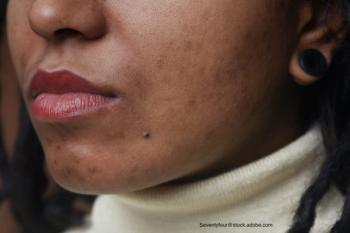
A letter to the editor in the Journal of the American Academy of Dermatology investigated how mental health and its association to race and ethnicity relate to patients with acne.
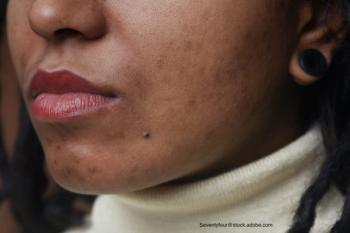
A letter to the editor in the Journal of the American Academy of Dermatology investigated how mental health and its association to race and ethnicity relate to patients with acne.

Impetigo is a common skin infection in which ozenoxacin may be used as a treatment. A study aimed to evaluate the safety and efficacy of the therapy in pediatric patients compared to other topical treatments.
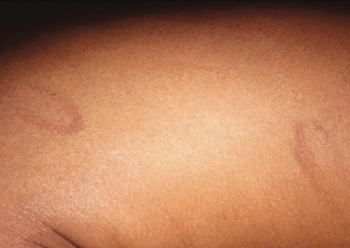
An 18-month-old girl presents with ringed scars with hyperpigmentation on the right side of her buttocks and back. What's the diagnosis?
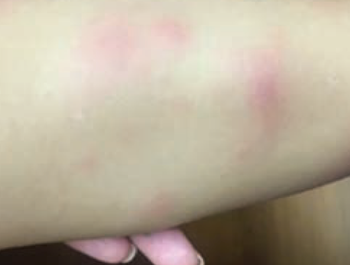
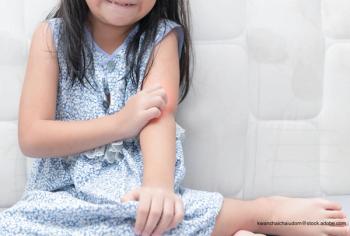
A 2-year-old girl presents with an itchy, bilateral leg rash. Additionally, the child had several bruises that felt like "hard welts" and were warm to the touch. What's the diagnosis?

A recent study examined the impact of virtual group visits to obtain community and education for pediatric vitiligo and alopecia areata patients and caregivers.

Seborrheic dermatitis will affect roughly half of all infants, making knowing how to treat and manage it key.

Parents who are struggling financially often try to stretch their diaper supply, which may increase the risk of a child developing diaper rash. Diaper banks are one place that can help.
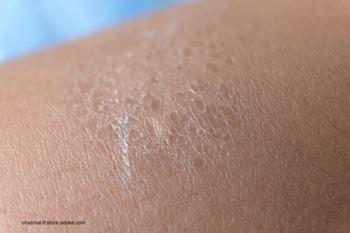
Research aimed to understand how using daily skin care such as a mild cleanser and moisturizer impacts skin health.

Skin cancer is on the rise. Knowing what teenagers and adolescents understand about protecting skin from the sun and how well they’re using that information can be important to changing that trend.

Experts discuss the impact of misinformation in medicine at the recent American College of Asthma, Allergy & Immunology annual scientific meeting.

A presentation from the 2021 virtual American Academy of Pediatrics Conference & Exhibition urges including examination of the scalp and nails during any skin exam.

An interview with Adelaide Hebert, MD, discusses how creams with ceramides can help manage atopic dermatitis (AD).
An interview with Adelaide Hebert, MD, how environmental factors may play a role in flare ups.

An interview with Adelaide Hebert, MD, about treating eczema in children, including tips on how often a child should bathe.

Most diaper rash requires strict hygiene and over-the-counter barrier creams. When dealing with more severe cases, there are a number of diagnoses and treatments to consider.

Dropping temperatures and dry air can wreak havoc on anyone's skin, but especially the delicate skin of an infant. Prevention is the best tactic to keep skin healthy.
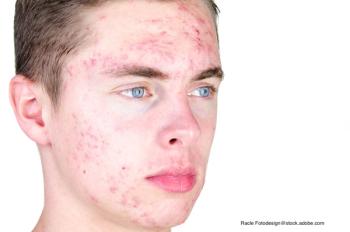
A study investigated the role diet habits have in the development, duration, and severity of acne.
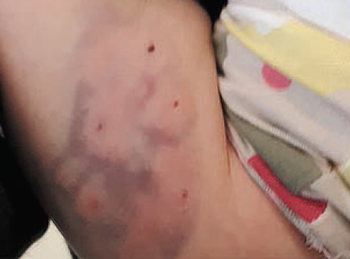
A neurodevelopmentally impaired 6-year-old girl is referred for examination of asymptomatic skin lesions on the right forearm due to concern for possible child abuse. What's the diagnosis?

In cases of poorly controlled disease, even with good medication adherence, it might make sense to turn to biologics. A presentation at the virtual 2021 American Academy of Pediatrics National Conference & Exhibition covered biologics available for treating asthma and allergic skin disease.

Treating dermatologic conditions in skin of color requires cultural awareness and sensitivity, in addition to being aware of how presentation may differ. At the virtual 2021 American Academy of Pediatrics National Conference & Exhibition, a session offered clinical pearls.
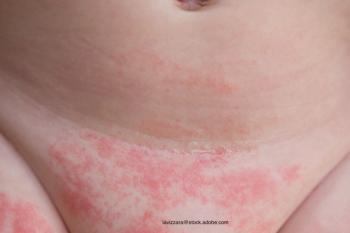
Managing an infant's diaper rash doesn't have to be difficult.

Cloth diapers are considered less likely to cause diaper rash, but that's not always the case.

Although not every case of diaper rash can be avoided, using preventive techniques can reduce damage.

Irritated skin in the diaper area may make parents first think of diaper rash, but atopic dermatitis is another possible condition. Knowing the triggers can help determine the correct diagnosis.
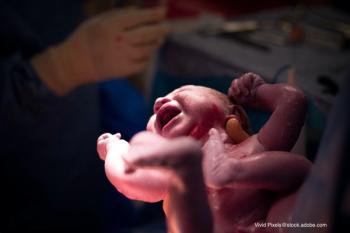
The first hours of life carry a long checklist, which often includes the newborn's first bath, but variation in the practice appears to be quite common across US birthing centers.