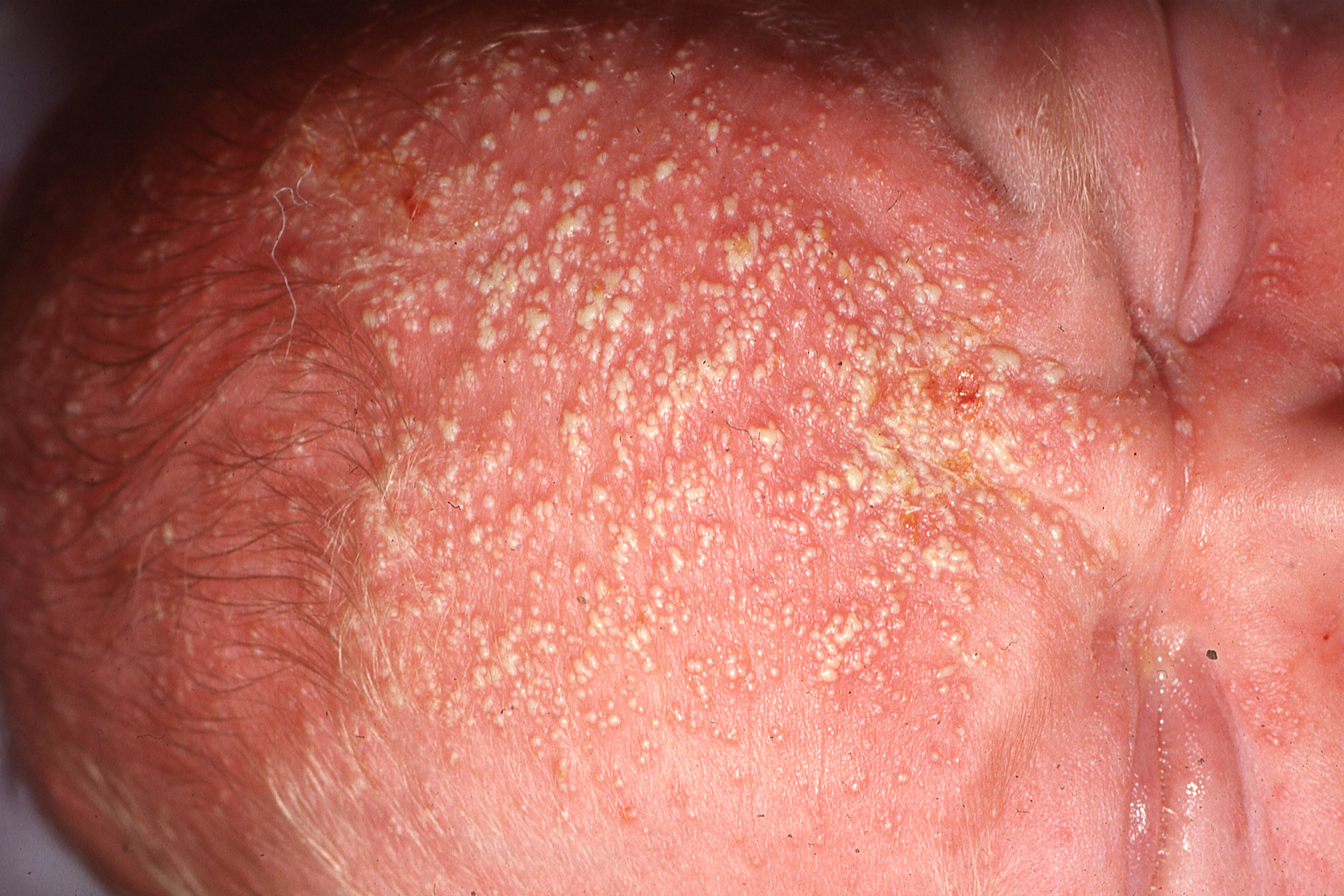
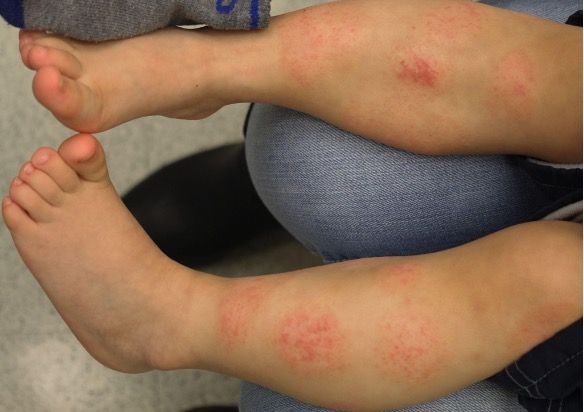
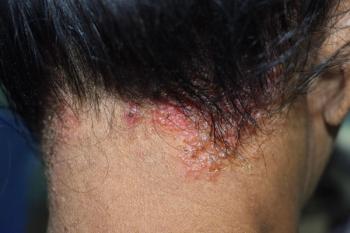
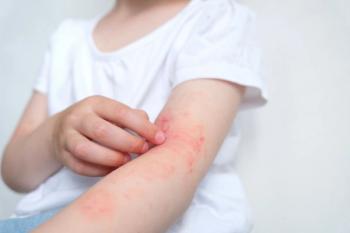
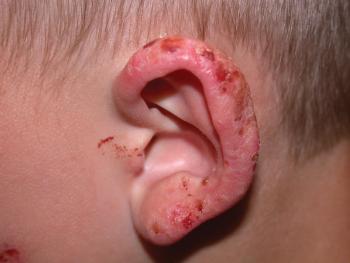
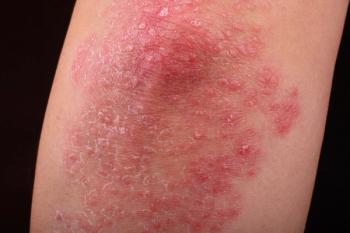
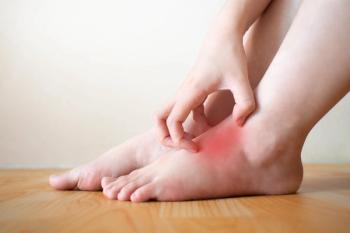
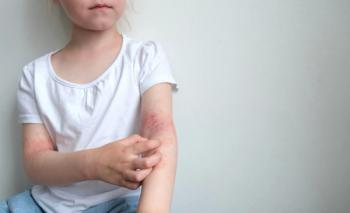
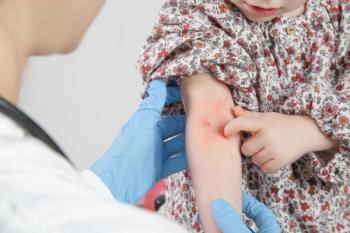
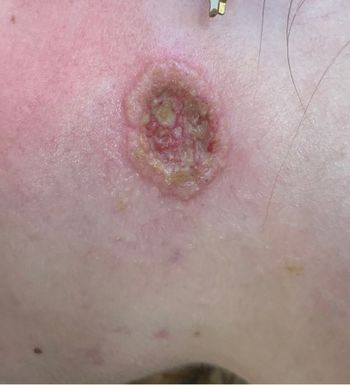
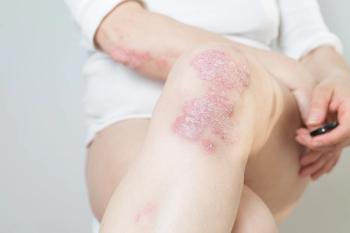
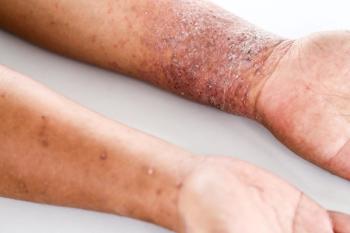
The once-daily topical cream for the treatment of atopic dermatitis (AD) has demonstrated positive topline results in 2 phase 3 studies in adults and children aged 2 years and up. According to Dermavant, a supplemental new drug application (sNDA) filed with the FDA is anticipated in Q1 of 2024.