
"I really hope this becomes a first-line agent that people are comfortable prescribing whether they are in dermatology, pediatric dermatology, or primary care."

"Tapinarof comes in with that mixture of the short-term studies and longer-term studies intermittently, giving us a nice, effective alternative non-steroid for eczema across the ages."

"I really hope this becomes a first-line agent that people are comfortable prescribing whether they are in dermatology, pediatric dermatology, or primary care."
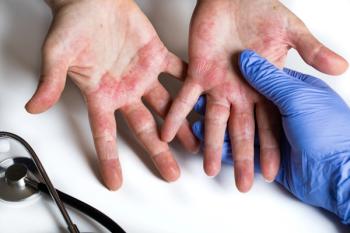
In the study through 76 weeks, no new risks were observed with once-daily upadacitinib (15 mg or 30 mg).
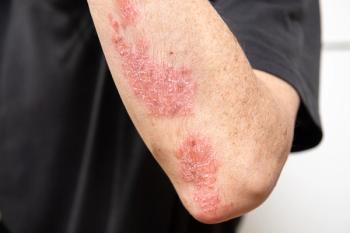
The submission to treat PsO is for children aged 6 years and up while the jPsA submission is to treat children aged 5 years and older.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the final Contemporary Pediatrics journal of the year.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from last week, all in one place.
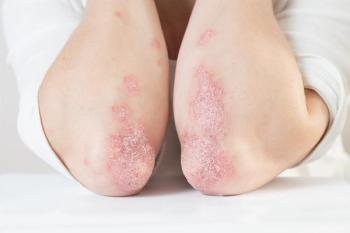
Once-daily icotrokinra versus placebo demonstrated clinically significant skin clearance in those with moderate-to-severe plaque psoriasis.

The application indication is for adults and pediatric patients aged 12 years and older with CSU whose disease is not adequately controlled with H1 antihistamines.

A target action date for potential approval of prademagene zamikeracel (pz-cel) has been set for April 29, 2025.

The federal agency has not raised any concerns regarding safety and efficacy of tapinarof cream, 1%.

In all, 51.9% of patients entered with or achieved complete disease clearance at least once during the 48-week study.
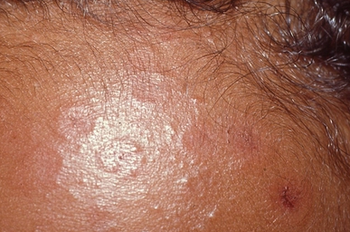
Can you diagnose this month's dermatology case?

A recent study presented at the American Academy of Pediatrics 2024 National Conference & Exhibition, sheds light on the connection between skin conditions and sleep disturbances in infants and toddlers, highlighting itchy skin as a significant factor, even in the absence of atopic

Eric Simpson, MD, MCR, FAAD, lead author of a recently-published article highlighting roflumilast cream 0.15%, joined us for a Q+A interview.

With this acceptance, the federal agency has assigned a PDUFA date of May 22, 2025 for roflumilast foam (Zoryve) 0.3%.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

The indication is supported by trial data showing three-fourths of successfully treated patients maintained clear skin through 1 year of a lebrikizumab regimen.
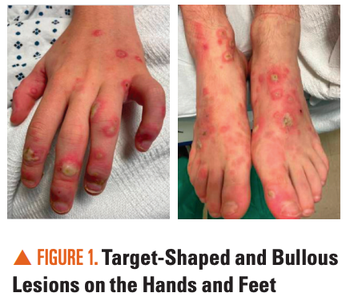
Can you diagnose this adolescent with an 11-day history of diffuse targetoid and bullous lesions on his extremities and trunk?

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the August 2024 issue of Contemporary Pediatrics.

Efficacy of roflumilast cream 0.05% improved over time among children aged 2 to 5 years with mild to moderate atopic dermatitis.

Donna Hallas PhD, PPCNP-BC, CPNP, PMHS, FAANP FAAN, highlights the August issue of our journal with commentary and recommendations.
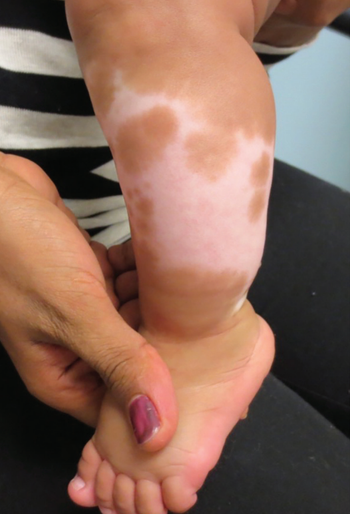
Standard treatment for vitiligo includes topical corticosteroids, topical calcineurin inhibitors, oral corticosteroids, and phototherapy, among others.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the August 2024 issue of Contemporary Pediatrics.

Initial approval for maralixibat was granted on March 13, 2024, to treat PFIC patients aged 5 years and older.

If approved, roflumilast foam 0.3% for scalp and body psoriasis treatment would add to the multiple already-approved roflumilast indications.