The primary endpoint of the study was to measure childhood stigma related to disease visibility.

The primary endpoint of the study was to measure childhood stigma related to disease visibility.

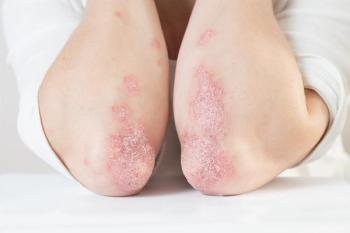
Once-daily, steroid-free, topical roflumilast cream 0.15% has a PDUFA date of July 7, 2024.

What are the skin conditions in which itching becomes the dominant clinical symptom in children?

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the June 2024 issue of Contemporary Pediatrics.

Wendy Ripple, MD, provides suggestions for sunscreens in the pediatric population.

As children head outside for summer, providers can remind parents of some staple sun safety tips.
These data from investigators at Trinity College Dublin highlighted the immune response mechanisms visible in children with atopic dermatitis.

Caregivers and parents should feel empowered to protect their children with sun-safe practices that include sunscreen, sun-protective clothing, and sun-safe activities.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the April 2024 issue of Contemporary Pediatrics.

According to new study data presented at the 2024 Pediatric Academic Societies Meeting, dupilumab (dupixent; Sanofi and Regeneron) demonstrated positive safety and efficacy results for up to 1 year in infants and preschool-age children with atopic dermatitis.

Data from a recent study revealed that stigma associated with chronic skin diseases made an impact on the quality of life of affected children and adolescents.

A decision from the federal agency is expected in the fourth quarter of 2024.

The biosimilar to ustekinumab is approved for patients 6 years and up, and is expected to be marketed on or after February 21, 2025.

Abeona's pz-cel is up for indicated use to treat patients with recessive dystrophic epidermolysis bullosa.
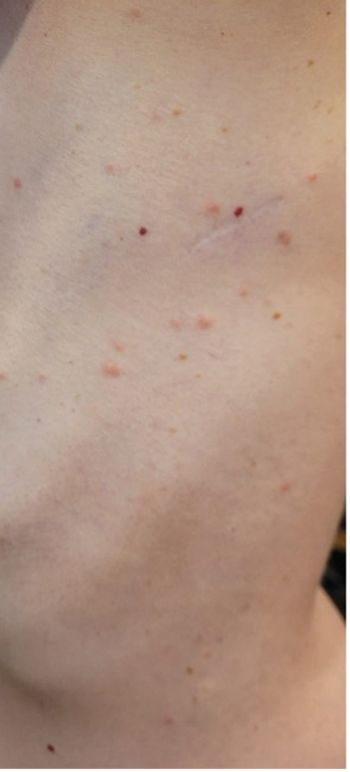
A young woman with no significant past medical history returns from hiking with several white-spotted ticks and experiences erythematous papules, rashes, headaches, and fatigue. What’s the diagnosis?
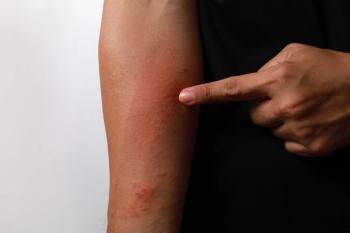
Data from a pair of identical, phase 3, double-blind, randomized, and vehicle-controlled trials were presented at the 2024 American Academy of Dermatology (AAD) Annual Meeting in San Diego, California.

Results from a recent laboratory test revealed benzene was found in multiple benzoyl peroxide acne products.

Health care providers must not only address the physical symptoms of patients with skin disorders but also the psychological fallout frequently associated with these diseases.

Lawrence Eichenfield, MD, highlights the sNDA submission of tapinarof cream, 1% for the topical treatment of atopic dermatitis in children aged 2 years and older.

The sNDA submission follows additional positive topline data that was presented in Janurary 2024, highlighting an open-label, long-term extension study evaluating tapinarof cream, 1%.
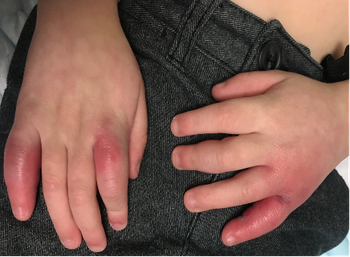
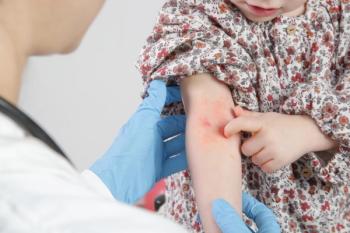
The child was otherwise healthy with no prior severe medical issues, and he did not present with COVID-19 symptoms. Can you diagnose this child based on the case below?

Read the case and take your best guess at diagnosing this 4-year-old patient.

In this Contemporary Pediatrics Q+A interview, Weily Soong, MD, breaks down FDA-approved tralokinumab-ldrm for patients aged 12 to 17 years with moderate-to-severe atopic dermatitis.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
According to new pooled individual patient responses, roflumilast cream 0.15% treatment led nearly 92% of individuals to achieve a measurable improvement in the Eczema Area and Severity Index.