Tapinarof cream 1% demonstrates positive topline results for treating pediatric AD
The once-daily topical cream for the treatment of atopic dermatitis (AD) has demonstrated positive topline results in 2 phase 3 studies in adults and children aged 2 years and up. According to Dermavant, a supplemental new drug application (sNDA) filed with the FDA is anticipated in Q1 of 2024.
Tapinarof cream 1% demonstrates positive topline results for treating pediatric AD | Image Credit: © arhat - © arhat - stock.adobe.com.
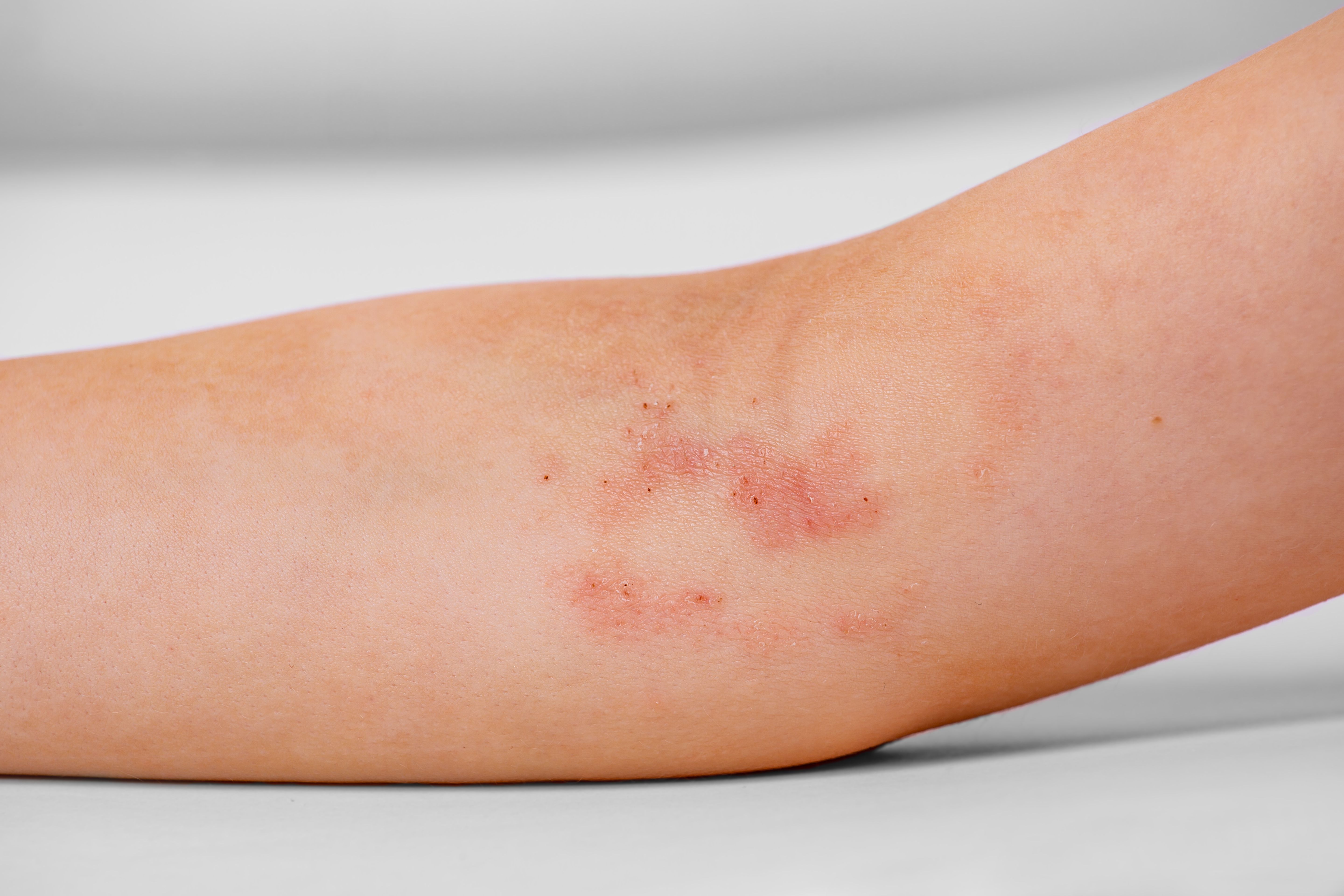
Tapinarof cream 1% (VTAMA; Dermavant) demonstrated positive topline results in the second of 2 phase 3 studies evaluating safety and efficacy while treating moderate to severe atopic dermatitis (AD) in patients 2 years and older, according to a press release from Dermavant.
According to Dermavant, tapinarof cream 1% is a novel, aryl hydrocarbon receptor agonist in development as a once-daily topical cream for the treatment of AD. Subject to FDA approval in AD, tapinarof is currently approved in the United States for the topical treatment of plaque psoriasis in adults.
The primary endpoint of the ADORING 1 (NCT05014568) phase 3 study was a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of clear (0) or almost clear (1) with at least a 2-grade improvement from baseline at week 8.
ADORING 1 (n = 407) is the second of 2 double-blind, randomized, vehicle-controlled phase 3 studies. ADORIDNG 1 met the primary and all key secondary endpoints, consistent with results from the ADORING 2 (NCT05032859) phase 3 trial, reported on in March, 2023, by Contemporary Pediatrics®.
In ADORING 1, adult and pediatric participants aged 2 years and up with moderate to severe AD were randomized (2 to 1) to receive once-daily tapinarof or vehicle cream. By week 8, 45.4% of participants that received tapinarof achieved the primary endpoint of a vIGA-AD score of 0 or 1 with at least a 2-grade improvement from baseline. In the trial, 55.8% of tapinarof users achieved the key secondary endpoint of the proportion of participants with a 75% or greater improvement in the Eczema Area and Severity Index (EASI75) from baseline at week 8 (P < 0.0001).
A significant improvement in itch was observed in study participants 12 years and older that received tapinarof. Of these participants with a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 4 or greater at baseline, 61.1% achieved a 4-point or greater reduction in the PP-NRS at week 8 (P = 0.0366), another key secondary endpoint of the study.
“Atopic dermatitis affects a significant number of children, and its prevalence continues to grow,” said Adelaide A. Hebert, MD, professor and chief of pediatric dermatology, McGovern Medical School at UTHealth Houston and Children’s Memorial Hermann. “The prevalence of itch as an associated symptom makes this condition extremely burdensome not only to the patients suffering from AD, but also their families. In this regard, the itch data from ADORING 1, much like that from ADORING 2, emphasizes [tapinarof]’s effectiveness when it comes to disease control and [tapinarof]’s potential to reduce one of atopic dermatitis’ most burdensome symptoms.”
According to the press release, adverse events were mild to moderate. The study discontinuation rate due to adverse events was 1.9% in tapinarof users and 3.6% in vehicle users. Contact dermatitis (1.5% tapinarof vs 2.2% vehicle) and follicular event (10.0% tapinarof vs 0.7% vehicle) were adverse events of special interest.
Tapinarof cream 1% was FDA approved for the treatment of adult psoriasis in 2022. According to Dermavant, a supplemental new drug application (sNDA) is anticipated to be filed with the FDA for atopic dermatitis treatment in Q1 of 2024.
Reference:
Dermavant reports positive topline results from ADORING 1, the second atopic dermatitis phase 3 trial of VTAMA (tapinarof) cream, 1% in adults and children as young as 2 years old. Dermavant Sciences. May 16, 2023. Accessed May 16, 2023. https://www.dermavant.com/dermavant-reports-positive-topline-results-from-adoring-1-the-second-atopic-dermatitis-phase-3-trial-of-vtama-tapinarof-cream-1-in-adults-and-children-as-young-as-2-years-old/

Newsletter
Access practical, evidence-based guidance to support better care for our youngest patients. Join our email list for the latest clinical updates.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.