- November 2021
- Volume 38
- Issue 11
2021 CDC vaccine schedule
A look at the Centers for Disease Control and Prevention's (CDC) vaccination schedule for the pediatric population.
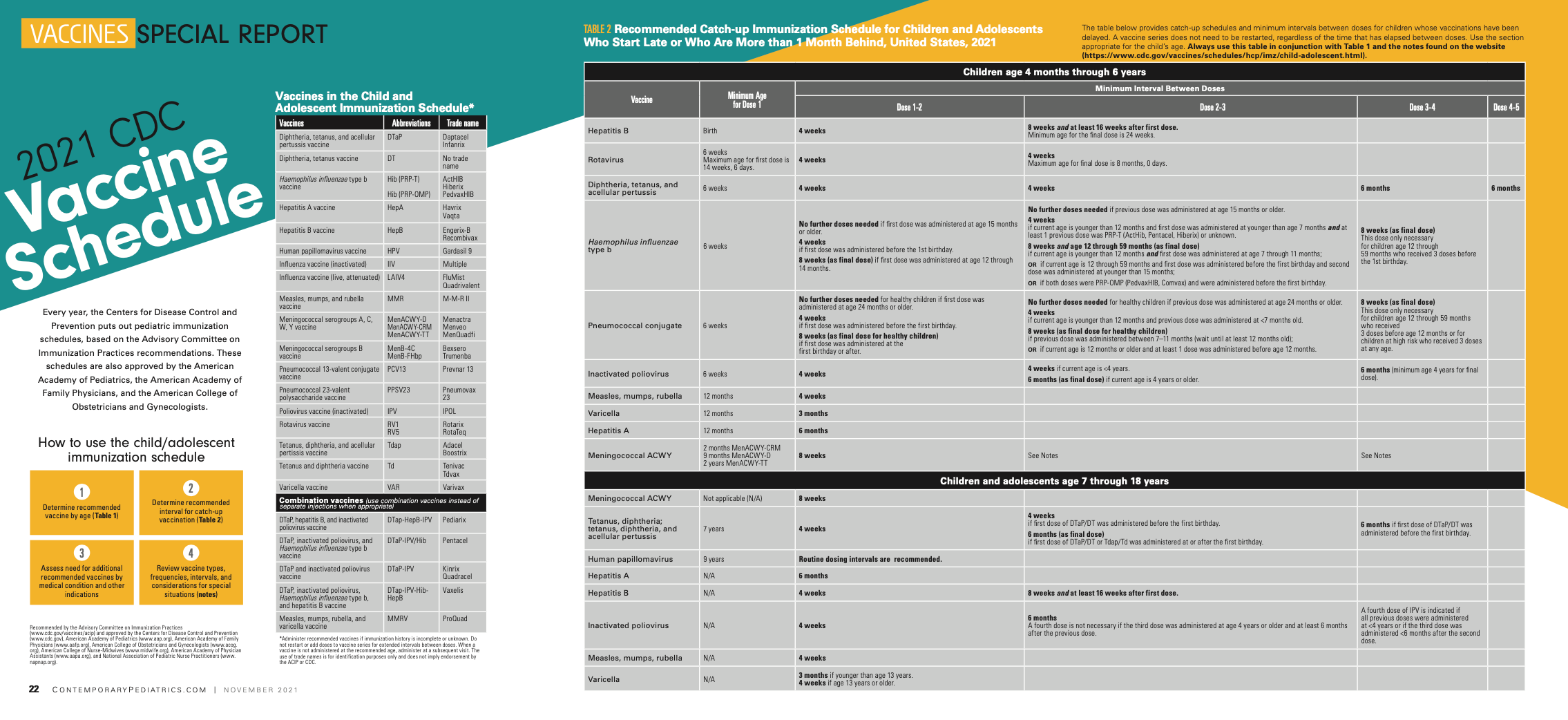
Every year, the Centers for Disease Control and Prevention puts out pediatric immunization schedules, based on the Advisory Committee on Immunization Practices recommendations. These schedules are also approved by the American Academy of Pediatrics, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists.
Notes: Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States, 2021*
For vaccination recommendations for persons ages 19 years or older, see the Recommended Adult Immunization Schedule, 2021.
COVID-19 Vaccination
ACIP recommends use of COVID-19 vaccines within the scope of the Emergency Use Authorization or Biologics License Application for the particular vaccine. Interim ACIP recommendations for the use of COVID-19 vaccines can be found at
• Consult relevant ACIP statements for detailed recommendations at
• For information on contraindications and precautions for the use of a vaccine, consult the General Best Practice Guidelines for Immunization at
• For calculating intervals between doses, 4 weeks = 28 days. Intervals of ≥4 months are determined by calendar months.
• Within a number range (eg, 12–18), a dash (–) should be read as “through.”
• Vaccine doses administered ≤4 days before the minimum age or interval are considered valid. Doses of any vaccine administered ≥5 days earlier than the minimum age or minimum interval should not be counted as valid and should be repeated as age appropriate. The repeat dose should be spaced after the invalid dose by the recommended minimum interval. For further details, see Table 3-1, Recommended and minimum ages and intervals between vaccine doses, in General Best Practice Guidelines for Immunization at
• Information on travel vaccination requirements and recommendations is available at
• For vaccination of persons with immunodeficiencies, see Table 8-1, Vaccination of persons with primary and secondary immunodeficiencies, in General Best Practice Guidelines for Immunization at
• For information about vaccination in the setting of a vaccine-preventable disease outbreak, contact your state or local health department.
• The National Vaccine Injury Compensation Program (VICP) is a no-fault alternative to the traditional legal system for resolving vaccine injury claims. All routine child and adolescent vaccines are covered by VICP except for pneumococcal polysaccharide vaccine (PPSV23). For more information, see
*This is not a complete list of notes. For the full set of notes, as well as additional tables, please see the CDC website at
Here's an easy-to-read PDF of the schedule:
Articles in this issue
about 4 years ago
Dr. Offit on COVID-19 vaccines for childrenabout 4 years ago
The future of vaccinologyover 4 years ago
The vaccine-hesitant patientover 4 years ago
Looking at the scalp and nails during a skin exam is keyover 4 years ago
Immunization information systems: Beyond the basicsover 4 years ago
The history of vaccinationover 4 years ago
Dedicated to vaccinesover 4 years ago
Noninvasive device accurately monitors Hb levelsNewsletter
Access practical, evidence-based guidance to support better care for our youngest patients. Join our email list for the latest clinical updates.