COVID-19
Latest News

Video Series
Latest Videos
Shorts

CME Content
More News
“While our study is small, its results are powerful and have implications not only for MIS-C, but potentially for long COVID,” said lead author Lael Yonker, MD.
The American Academy of Pediatrics will continue to hold its own childhood vaccine schedule, as it has since the 1930s.
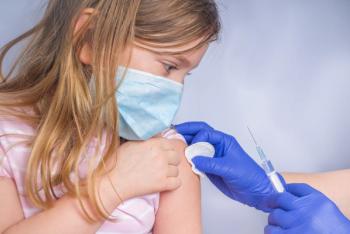
In this article, we recap a timeline of recent federal agency changes to routine COVID-19 vaccination intended for the pediatric population.

Federal health agencies have adjusted COVID vaccine guidance, potentially changing eligibility and access for pediatric patients. Experts respond in this article.

Infants and toddlers with infection history were more likely to have trouble sleeping, while preschool-aged children experienced daytime tiredness.
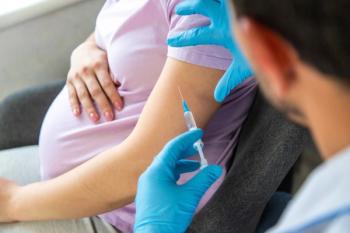
RFK Jr. stated he "couldn't be more pleased" to announce that COVID-19 vaccination among healthy children and pregnant women has been removed from CDC's immunization schedule.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

NVX-CoV2705 is indicated for individuals aged 12 to 64 years with an underlying condition that poses high risk for severe COVID-19 outcomes.

Mike Hennessy Jr, President and CEO of MJH Life Sciences kicks off our March Mental Health issue with a look into the ongoing pediatric mental health crisis.

This week the panel weighs in on monoclonal antibodies in this patient population as well as important overall takeaways about COVID-19 therapies.
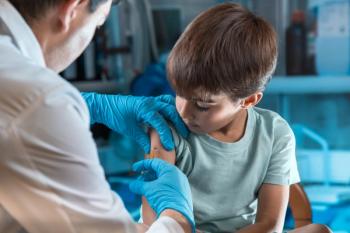
The most common reactions reported were the following: irritability or fussiness (30.1%), local reactions (21.1%) and fever (13.8%).

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from last week, all in one place.
A further understanding of the role SARS-CoV-2 plays in T2D incidence can add an important component to benefit and risk considerations to prevent COVID-19.
Donna Hallas, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, highlights key takeaways from our October Vaccine issue of Contemporary Pediatrics.

A single dose of the mRNA-1273.222 vaccine demonstrated strong immune responses against various omicron subvariants and showed a favorable safety profile compared to existing vaccines.

The federal agency advised manufacturers in June that 2024-2025 COVID-19 vaccines should be monovalent JN.1, with the preferred lineage being the KP.2 strain.

Data from an Italy cohort show wheezing episodes decreased by more than 40% among children born during the COVID-19 lockdown period versus a pre-pandemic cohort.

Matthew M Davis, MD, MAPP, elaborates on how higher COVID-19 vaccination rates may curb the virus, alleviate childhood asthma symptoms, and protect against other common colds.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
Over the winter, the omicron JN.1 lineages overtook XBB strains as dominant.

New cohort analyses observe a statistically raised risk of myocarditis, pericarditis and seizures among certain age groups, though the data do not go against FDA recommendations.

An assessment into development scores before, during and after the COVID-19 pandemic show children aged 0 - 5 years old may only had been minimally impacted in communication and problem-solving skills.

This analysis looked at the potential connection between SARS-CoV-2 test positivity and new asthma diagnoses.

Of the 600,238 bivalent vaccine doses administered by all providers in the study period, 35,114 (5.9%) were done so by FRPP partners.
New data demonstrates protection against new variants.