
In a recent report, the American Academy of Pediatrics discussed how influenza affects children and how it can be prevented.

In a recent report, the American Academy of Pediatrics discussed how influenza affects children and how it can be prevented.
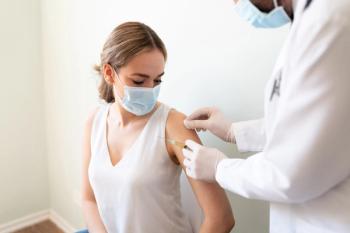
In a recent study, participants who had received a COVID-19 vaccine saw an average increase in menstrual cycle length of less than 1 day.
Dr Muller and Dr Offit provide take-home messages about COVID-19 vaccines for pediatric patients.

Expert pediatricians discuss challenges with coadministration of vaccines in children younger than 5 years.
In 2 new education documents, Physicians for Informed Consent outlines the risks of Hepatitis B compared to the risks of the Hepatitis B vaccination.
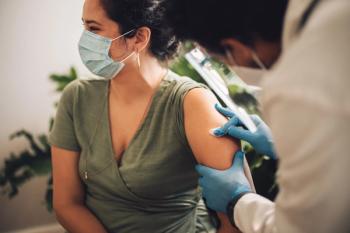
Infants of fully vaccinated mothers had fewer cases of hospitalization from COVID-19 than those unvaccinated.
Dr Muller and Dr Offit discuss recent developments in the COVID-19 vaccine for children younger than 5 years.

Dr Muller and Dr Offit discuss COVID-19 variants and their effect on vaccine development.
Move comes after FDA give emergency use authorization for bivalent vaccines.
In a recent study, investigators found lower rates of COVID-19 infection and other adverse outcomes in vaccinated pregnant people over those unvaccinated.

Latest emergency use authorizations for Moderna, Pfizer-BioNTech vaccines.

Paul A. Offit, MD, comments on spread of the COVID-19 virus in children who are vaccinated.

Vaccine and infectious disease experts address vaccine hesitancy and the importance of the COVID-19 vaccine in children.

William J. Muller, MD, PhD; and Paul A. Offit, MD, discuss the impact of COVID-19 on children.

Drs William J. Muller and Paul A. Offit discuss differences in response to vaccines in children compared to adults.

Novavax joins the growing list of COVID-19 vaccines including vaccines from Pfizer-BioNTech, Moderna, and Johnson & Johnson’s Janssen

The news is full of stories about monkeypox, the recent changes to COVID-19 guidance, and the return of polio cases. What do clinicians need to know?
Only one-fifth of parents in a recent survey indicated intentions of getting their child the vaccine within 3 months of eligibility.
Learn about the selected strains for this year’s flu season, flu vaccine recommendation changes, and newly licensed and forthcoming vaccine options.
As immunization rates fall in children, the American Academy of Pediatrics recommends a checkup with physicians.
Purchase follows FDA recommendation to include Omicron component in boosters.

In children 5-11 years old, 2 doses of the Pfizer-BioNTech COVID-19 vaccine were moderately effective against documented, symptomatic Omicron infection.
Patients with a history of infection who were also vaccinated had the strongest protection, the data showed.
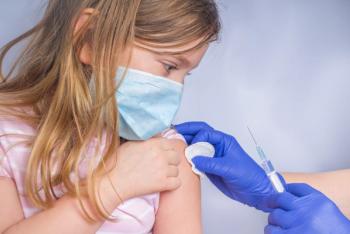
Recently, the AAP announced the launch of a public health campaign to educate caregivers on the benefits of vaccinating all ages.

The FDA today approved the pneumococcal 15-valent conjugate vaccine for the prevention of invasive pneumococcal disease in infants and children.