Vaccines
Latest News
Latest Videos

CME Content
More News

The program allows participants to reserve doses and be eligible for priority shipping, among other benefits.

Courtney Nelson, MD, highlights the session she presented at the PAS annual meeting discussing racial and ethnic disparities for influenza vaccine delivery.

In a time when vaccine hesitancy is contributing to a rise in measles cases, an understanding of why parents are hesitant is key to help change their narrative.

The 5-in-1 vaccine candidate has the potential to reduce shots and simplify the immunization process.
The monoclonal antibody was approved by the FDA on July 17, 2023 and quickly saw high demand at the onset of the RSV season.
A study on Italian vaccination rates and factors influencing Rotavirus vaccine acceptance.
Of the 600,238 bivalent vaccine doses administered by all providers in the study period, 35,114 (5.9%) were done so by FRPP partners.
New data demonstrates protection against new variants.

Mary Koslap-Petraco, DNP, PPCNP-BC, CPNP, FAANP, provided a review and reminder of the CDC immunization recommendations and schedule during her session at the 45th National Association of Pediatric Nurse Practitioners (NAPNAP) National Conference on Pediatric Health Care in Denver, Colorado.

An intervention applied at family care practices shows benefit to tailoring education to both clinicians and medical assistants.

Though a small number of infants received nirsevimab in the analysis, results support existing nirsevimab recommendations to prevent serious RSV disease in infants.

Samantha Olson, MPH, explains the important role of a strong recommendation for maternal influenza vaccination.

Samantha Olson, MPH, breaks down a study highlighting the effectiveness of maternal influenza vaccination and its respective association with influenza-related hospitalizations in infants.

Nirsevimab was approved by the FDA on July 17, 2023, ahead of the traditional RSV season, though in October, the Centers for Disease Control and Prevention (CDC) recommended it be prioritized for the highest-risk infants amid limited availability.
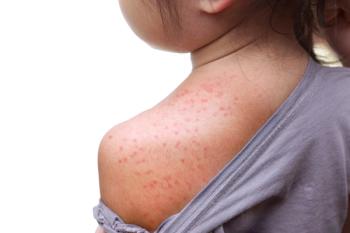
Tina Tan, MD, FAAP, FIDSA, FPIDS, tells Contemporary Pediatrics, “This is not new and demonstrates what is known, in that if vaccination rates do not stay at a level that is protective, outbreaks of vaccine preventable diseases will occur.”
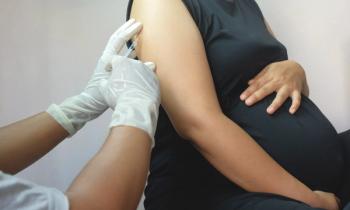
Investigators of a new study published in JAMA Pediatrics concluded that maternal vaccination was safe with regard to neurodevelopment of the child at ages 12 months and 18 months.
Todd Wolynn, MD, stresses the need for effective communication within the pediatric physician community to combat misinformation, particularly about influenza vaccination.

An expert pediatrician reviews vaccine coverage rates within the pediatric population, and addresses parents’ and guardians concerns when it comes to vaccinating children against influenza.

Investigators of a study published in Annals of Internal Medicine concluded that the BNT162b2 COVID-19 vaccine demonstrated efficacy against the Delta and Omicron variants of COVID-19 in children and adolescents, offering new, extended follow-up period data.

Todd Wolynn, MD, continues the discussion on egg-based vs cell-based influenza vaccines in pediatric populations, commenting on the limitations and concerns with traditional egg-based formulations.

Todd Wolynn, MD, reviews influenza vaccination recommendations for pediatric populations, highlighting the egg-based and cell-based formulations.
The estimated efficacy of the vaccine against documented infection for children and adolescents during the Omicron wave was 74.3% and 85.5%, respectively.
A recently published study on 17 million children found combined flu and COVID-19 vaccines offered superior protection against hospitalization, MIS-C, and death during the pandemic.
Overall, the study authors concluded that the immunogenicity of QIV and TIV was similar for common influenza ingredients for children aged 6 to 35 months.

The US approval makes VLA1553 (Ixchiq; Valneva) the world’s first licensed chikungunya vaccine.