
Gastroenterology
Latest News
Latest Videos

CME Content
More News

The federal agencies are encouraging companies to develop new infant formulas and clarify opportunities to inform consumers about formula ingredients.

On World Obesity Day, take this short quiz and test your knowledge of the AAP's clinical practice guideline to evaluate and treat children and adolescents with obesity.

Colleen Sloan, PA-C, RDN, emphasizes the importance of learning to spot added sugars, compare portion sizes, and recognize misleading marketing claims to give patients a practical tool to take ownership of their health.

The human, pasteurized human milk fortifier is approved for term infants (> 37 weeks) following corrective surgery for the gastrointestinal disorder gastroschisis.

The designations follow the FDA acceptance of IND application for ABO-101 to treat PH1, with a phase 1/2 study planned in the first half of 2025.

According to a new study, racial and ethnic disparities were evident when counseling on nutrition, lifestyle, and weight among children with high blood pressure measurements.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the January/February, 2025, issue of Contemporary Pediatrics.

"The greatest risk reductions were demonstrated in those who experienced obesity remission during childhood," stated study authors.

The digestive enzyme cartridge is designed to mimic pancreatic lipase function.

Across all years in the report, the CDC rated each physical activity trend with a color of red, indicating that trends are going in the "wrong direction."

Colleen Sloan, PA-C, RDN, explores how diet can help manage childhood constipation.

A recent study highlights potential reductions in macronutrient levels in breast milk because of maternal medications, though breastfeeding remains strongly recommended.

The expanded indication is approved to reduce excess body weight and maintain reduction long-term in children 2 years and up with obesity due to BBS, POMC, or LEPR deficiency.

"Our findings support the recommendation of a healthy diet based on the current guidelines (as measured by the HEI) during pregnancy, since it may reduce patterns of infant growth outside reference ranges."

Currently, golimumab is approved in adults with moderately to severely active ulcerative colitis.
A decade after bariatric surgery, most teens maintained weight loss and reduced obesity-related conditions such as type 2 diabetes and hypertension.

The FDA, CDC, and NIH have released a consensus statement regarding premature infant nutrition and necrotizing enterocolitis.

While rates of exclusive breastfeeding have slightly increased between 2016 and 2022, they remain under the Healthy People 2030 goal.

In this article, Colleen Sloan explores key nutrition strategies that pediatricians can confidently share with families to help their young athletes perform at their best.

Kay Rhee, MD, discusses the challenges of pediatric obesity treatment, highlighting the role of biological and environmental factors, behavioral interventions, and the potential benefits of GLP-1 medications in weight management for children and teens.

In her September 2024 article, Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, highlights the potential of digital health tools to improve care for pediatric mental health, obesity, and medically complex conditions.

Investigators found the association for higher BMI, increased obesity risk, and increased severe obesity risk.

A pediatrician's guide to the new baby food safety regulations from Tanya Altmann, MD, FAAP.
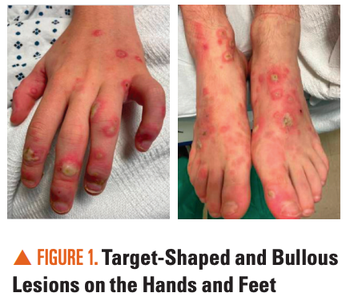
Can you diagnose this adolescent with an 11-day history of diffuse targetoid and bullous lesions on his extremities and trunk?

"The quest for a cure for colic continues," noted Jon Matthew Farber, MD.

















