
The landmark indication is supported by a couple of pivotal trials assessing dupilumab for patients with chronic rhinosinusitis with nasal polyps, as well as a pediatric severe asthma trial assessing the biologic.

The landmark indication is supported by a couple of pivotal trials assessing dupilumab for patients with chronic rhinosinusitis with nasal polyps, as well as a pediatric severe asthma trial assessing the biologic.

Biogen's Phase 2/3 study showed a higher dose of nusinersen improves motor function in SMA infants, with plans for regulatory submission to advance treatment.

Availability for the type 1 allergic reactions treatment approved by the FDA in August is expected later this month.

The investigational MEK inhibitor has been assigned a Prescription Drug User Fee Act (PDUFA) date of February 28, 2025.

Efficacy of roflumilast cream 0.05% improved over time among children aged 2 to 5 years with mild to moderate atopic dermatitis.

Following the FDA's acceptance of the NDA, a PDUFA date of December 26, 2024 has been set for setmelanotide in patients as young as 2 years of age.

The federal agency advised manufacturers in June that 2024-2025 COVID-19 vaccines should be monovalent JN.1, with the preferred lineage being the KP.2 strain.

The investigational drug demonstrated promise in mitigating hemolytic disease of the fetus and newborn by blocking harmful antibodies.

The FDA approved anacaulase-bcdb for pediatric use, allowing its application for eschar removal in deep thermal burns for ages newborn to 18 years.

The approval is indicated for adult and pediatric patients who weigh at least 88 lbs (40 kg).

Lynn Malec, MD, joined us to discuss the phase 3 XTEND-Kids study of ALTUVIIIO in pediatric patients with hemophilia A.

The approval is indicated for adult and pediatric patients who weight at least 66 pounds (30 kilograms).

Current treatment is primarily supportive care consisting of increased fluid intake to dilute oxalate in the urine, along with pyridoxine, or vitamin B6, to reduce oxalate production.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
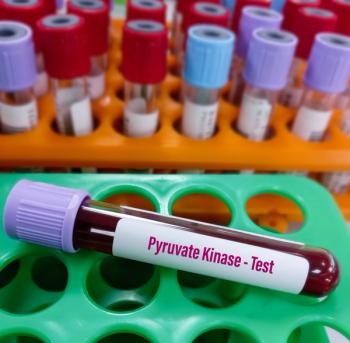
Nine of 32 (28.1%) patients in the mitapivat arm achieved a transfusion reduction response compared to 11.8% of patients in the placebo arm.

These data highlighted new findings on the experiences of children aged 6 to 11 with uncontrolled type 2 asthma treated with dupilumab.

With the expanded label, Palforzia is now approved to treat individuals aged 1 to 17 years with a confirmed peanut allergy diagnosis, after the treatment was originally approved in 4-to-17-year-olds in January 2020.

ConSynance Therapeutics' CSTI-500, an innovative Triple Monoamine Reuptake Inhibitor, has received FDA's Rare Pediatric Disease Designation for treating Prader-Willi Syndrome in children.

From baseline to final assessment, all 7 matched cerliponase alfa-treated children under 3 years of age maintained a motor score of 3, representing a "grossly normal gait, signifying a delay in disease onset," stated BioMarin.

Initial approval for maralixibat was granted on March 13, 2024, to treat PFIC patients aged 5 years and older.

Merck announced clesrovimab met all primary safety and efficacy endpoints, with additional detailed findings to be presented at an upcoming scientific congress.

With the acceptance of the BLA, a new PDUFA date of January 7, 2025 has been set.

If approved, roflumilast foam 0.3% for scalp and body psoriasis treatment would add to the multiple already-approved roflumilast indications.

The Rare Pediatric Disease Designation from the FDA follows the previously granted Orphan Drug and Fast Track Designations.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.