
Drug Pipeline News
Latest News

CME Content

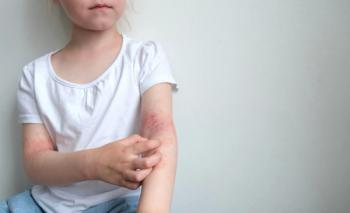
The investigational biologic targets the interleukin-2 receptor complex in the body in order to stimulate proliferation of inhibitory immune cells.

Cyclo Therapeutics has enrolled 10 patients in a single-arm sub-study treating newborns to 3 years of age, evaluating the Trappsol Cyclo in the youngest subsets.

The designations follow the FDA acceptance of IND application for ABO-101 to treat PH1, with a phase 1/2 study planned in the first half of 2025.

A recap of the FDA submissions and regulatory decisions in pediatrics from January 2025.
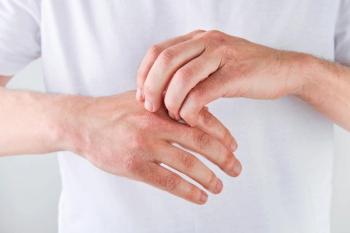
The primary and all key secondary endpoints were met compared to cream vehicle in the phase 3 DELTA TEEN trial.

Application submission is based on positive phase 3 data that demonstrated improvement in patients with type 2 and 3 SMA receiving current standard of care.

The higher dose regimen of nusinersen comprises a more rapid loading regimen, two 50 mg doses 14 days apart, and higher maintenance regimen, 28 mg, every 4 months.

The digestive enzyme cartridge is designed to mimic pancreatic lipase function.

The lead clinical trial investigator for a phase 3 study of crinecerfont in pediatric CAH patients breaks down the December 2024 FDA approval.

Neffy was first approved in September 2024 to treat type 1 allergic reactions in patients who weigh at least 66 lbs (33 kg).

Positive interim data was reported from the ENERGY 1 trial and Expanded Access Program that featured a total of 5 infants and 1 child.

Contemporary Pediatrics' editorial advisory member Russell Libby, MD, FAAP, highlights 2024 FDA approval of epinephrine nasal spray (Neffy) in this video interview.

Needle-free treatments, such as Neffy and FluMist, offer promising alternatives for children and adults with needle phobia, ensuring access to critical care.

The indication is for patients 12 years and older hemophilia A with factor VIII inhibitors or hemophilia B with factor IX inhibitors.

The FDA has approved the first generic once-daily GLP-1 injection for the improvement of glycemic control for type 2 diabetes in patients 10 years and up.

The expanded indication is approved to reduce excess body weight and maintain reduction long-term in children 2 years and up with obesity due to BBS, POMC, or LEPR deficiency.

Lawrence Eichenfield, MD, talks tapinarof cream, 1%, nemolizumab FDA approvals for atopic dermatitis
"Tapinarof comes in with that mixture of the short-term studies and longer-term studies intermittently, giving us a nice, effective alternative non-steroid for eczema across the ages."

The primary endpoint was non-inferiority in HbA1c levels after 26 weeks.

With the federal agency's decision, remestemcel-L-rknd has become the first FDA-approved mesenchymal stromal cell therapy.

From new topical dermatology treatments for atopic dermatitis to the first nasal spray to treat type 1 allergic reactions, these are our top FDA approvals of 2024.

Currently, golimumab is approved in adults with moderately to severely active ulcerative colitis.

The federal agency has set a target action date of June 10, 2025 for potential approval.

Arcutis seeks FDA approval for roflumilast cream 0.05% (ZORVYE) to treat mild to moderate atopic dermatitis in children 2 to 5 years.

The FDA has approved Crenessity for managing classic CAH in patients 4 years and up, reducing steroid doses while controlling hormones, offering a safer treatment option.






