
The approval marks a vital step toward care for urinary tract infections, helping to reduce recurrence and improve patients’ quality of life.

The approval marks a vital step toward care for urinary tract infections, helping to reduce recurrence and improve patients’ quality of life.

Results were from the phase 3 STEER study among a broad population of patients with SMA aged 2 to under 18 years.

Prior to approval on February 14, 2025, the only FDA approved vaccine for chikungunya protection was indicated for 18 years and up.

In the final part of this 5-part series, panelists discuss new allergy treatments such as Xolair, early immunotherapy, and their broader impact.

In part 4 of this 5-part series, panelists discuss gaps in pediatric food allergy care, stressing better diagnosis, education, and management.

In part 3 of this 5-part series, panelists discuss the recent Neffy approval, oral immunotherapy, and Xolair’s role in allergy management.

In part 2 of this 5-part series, panelists discuss confusion in food allergy diagnosis and the need for better medical education.

In part 1 of this 5-part series, panelists discuss early allergen introduction, oral immunotherapy, and Xolair for food allergy.

If approved, SYD-101 would be the first and only pharmaceutical option for the treatment of progression of pediatric myopia in the United States.

Jay T. Rubinstein, MD, PhD, highlighted promising CHORD trial data showing that DB-OTO gene therapy has led to hearing improvements in treated participants.

The newly-approved indication is for children aged 4 years and older who weigh 33 < 66 lbs, expanding on the August 9, 2024 approval in children at least 66 lbs.

First approved for PNH in 2007, the monoclonal antibody is now the first and only treatment for pediatric patients living with gMG.

Results will be included an a final analysis of safety and efficacy data for CTx-1301, which will be included in a NDA submission with the FDA, stated Cingulate Inc.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The expanded indication now includes children aged 5 through 11 years, in addition to patients aged 12 to 65 years.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from February 2025.

Palforzia [Peanut (Arachis hypogaea) Allergen Powder-dnfp] has now launched in the US for children aged 1 through 3 years with a confirmed diagnosis of peanut allergy.

The human, pasteurized human milk fortifier is approved for term infants (> 37 weeks) following corrective surgery for the gastrointestinal disorder gastroschisis.

Updated data from the phase 1/2 CHORD trial in 12 children with profound genetic hearing loss were presented at the Association for Research in Otolaryngology’s 48th Annual MidWinter Meeting.
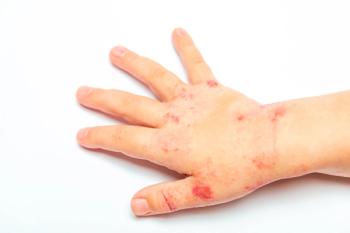
Nearly 40% of children treated with roflumilast cream 0.05% achieved a 75% improvement in EASI-75.

The FDA has accepted PTC Therapeutics' NDA for vatiquinone, a potential treatment for Friedreich's ataxia, with a decision expected by August 19, 2025.

90-day interim biopsy data in the first 3 patients revealed an average microdystrophin expression of 110% and improvements in muscle health and resilience biomarkers.

Nurse practitioner Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN offers her thoughts on the recent FDA approval of GSK's meningococcal ABCWY vaccine.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The combination vaccine targets 5 groups of the bacteria Neisseria meningitidis (A,B,C,W, AND Y) that cause the most invasive meningococcal disease cases (IMD) globally.