
Drug Pipeline News
Latest News
Latest Videos

Shorts

CME Content
More News

The agency approved a monthly dosing option for select patients acorss all approved indications, including wAMD, DME, DR, and RVO.

“This label expansion helps close a long-standing gap in the treatment of pediatric patients with hereditary antithrombin deficiency,” said George M. Rodgers, III, MD, PhD.

The target action date for roflumilast cream 0.3% for plaque psoriasis in children 2 to 5 years is set for June 29, 2026.

Long-term data show sustained survival, immune recovery, and metabolic correction after lentiviral gene therapy for ADA-SCID, with no vector-related safety events.

FDA adds boxed warning to Elevidys and limits use to ambulatory DMD patients ≥4 years after reports of fatal acute liver failure in non-ambulatory patients.
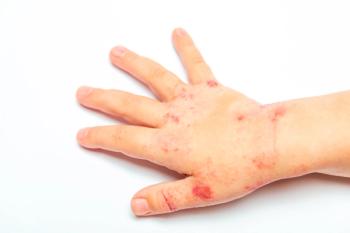
Sub-analysis of ADORING trials shows VTAMA cream improved skin clearance, symptoms, and patient-reported outcomes in children aged 2-17.

Dupilumab maintained histologic and endoscopic improvements across all pediatric age groups with EoE, according to Joshua Wechsler, MD.

Thomas Wallach, MD, discusses phase 3 safety data of tenapanor in pediatric patients with IBS-C, presented at the 2025 NASPGHAN Annual Meeting.

Long-term analysis of the PEDFIC studies presented at NASPGHAN 2025 demonstrated sustained reductions in bile acids and pruritus with odevixibat in FIC1 deficiency.

Julie Khlevner, MD, AGAF, explains the impact of the FDA approval of linaclotide for pediatric IBS-C
Julie Khlevner, MD, AGAF, highlights that FDA approval of linaclotide provides the first pharmacologic therapy for pediatric IBS-C.

At NASPGHAN 2025, investigators presented 52-week safety data from an ongoing phase 3 trial of linaclotide, the first approved treatment for patients aged 7 and older with IBS-C.

FDA approves first irritable bowel syndrome with constipation (IBS-C) treatment, linaclotide (Linzess), for children aged 7 years and older based on pediatric and adult trial data.

FDA approves Kygevvi, the first therapy for thymidine kinase 2 deficiency, a rare mitochondrial disease marked by muscle weakness and early death.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from October 2025.

If approved, this UC indication would add to pediatric indications of ustekinumab for psoriasis and active psoriatic arthritis.

FDA moves to remove unapproved ingestible fluoride drugs for children, citing microbiome and thyroid concerns.

The FDA has assigned a target action date of February 28, 2026, for a potential expanded indication of pegvaliase-pqpz to include adolescents with PKU.

A pair of phase 3 trials showed that AbbVie's daily upadacitinib 15 mg led to a 50% reduction in T-VASI 50 and a 75% reduction in F-VASI 75 at 48 weeks among adolescents 12 years and older.

New one-year data showed that baricitinib continued scalp, eyebrow, and eyelash regrowth in adolescents with severe alopecia areata.

The FDA acknowledged that the primary endpoint was met but that "data do not support the effectiveness of low-dose atropine in children with myopia," according to Sydnexis.

Tezepelumab received FDA approval for CRSwNP in patients 12 years and older, supported by strong phase 3 data.

Indication expansions include hidradenitis suppurativa for patients 12 years and up, as well as for patients with uveitis aged 2 years and older.

FDA accepts MannKind’s sBLA for Afrezza in children and adolescents with diabetes.

A pair of roflumilast clinical trial investigators react to the FDA approval of the 0.05% formulation to treat atopic dermatitis in children aged 2 to 5 years.

The once-daily, steroid-free cream can be used anywhere on the body for treatment and for any duration, according to Arcutis.













