Drug Pipeline News
Latest News
Latest Videos
Shorts









CME Content
More News

Three-year EMBARK data show ELEVIDYS slows functional decline in ambulatory Duchenne patients, extending earlier 2-year efficacy findings.

FDA accepted centanafadine NDA for priority review, supported by phase 3 trials showing efficacy in children, adolescents, and adults with ADHD.

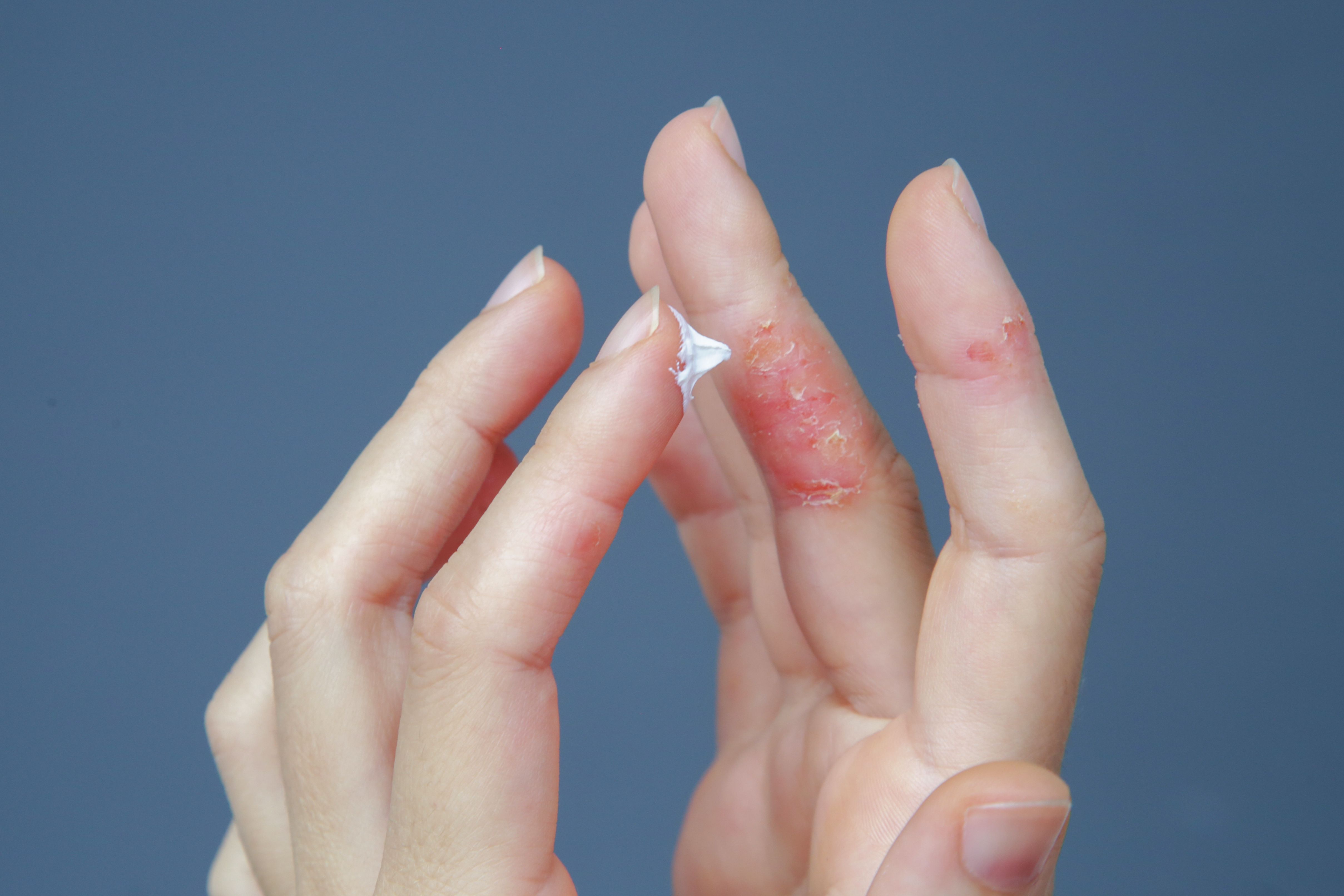
Findings from phase 3 SHORE and COAST 2 trials showed amlitelimab met key efficacy end points in moderate to severe atopic dermatitis, supporting planned regulatory submissions.

FDA clears first at-home test to detect RSV, influenza, and COVID-19 in infants as young as 6 months
FDA-cleared Flowflex Plus 4-in-1 home test detects RSV, influenza A/B, and COVID-19 in adults and children as young as 6 months.

Vimal H. Prajapati, MD, discusses the latest roflumilast cream data from the INTEGUMENT-PED trial for pediatric atopic dermatitis.

New phase 3 trial data reveal fremanezumab's effectiveness and safety for preventing migraines in children, offering a vital treatment option.

Fremanezumab-vfrm (Ajovy) reduces migraine frequency in children, adolescents with episodic migraine
The phase 3 SPACE trial showed fremanezumab reduced monthly migraine days vs placebo in pediatric patients with episodic migraine.

Phase 3 SCOUT-HCM results showed mavacamten significantly reduced LVOT gradient vs placebo in adolescents with obstructive HCM.

This recent FDA clearance expands the use of the iotaSOFT Insertion System for pediatric use.

The approval marks the first treatment for this neurodegenerative with a low survival rate beyond 3 years.

The Phase 3 Beeline trial has begun dosing patients to evaluate radiprodil, a targeted NMDA receptor modulator designed to address the underlying biology of GRIN-related neurodevelopmental disorder.

Once-weekly navepegritide plus lonapegsomatropin demonstrates growth in children with achondroplasia
Findings from a phase 2 trial showed that once-weekly TransCon CNP plus TransCon hGH produced durable growth and proportionality benefits in children with achondroplasia.

Results from a large randomized trial found that a midpregnancy blood test paired with targeted interventions improved neonatal outcomes in low-risk pregnancies.

FDA approved caplacizumab for pediatric patients ≥12 years with acquired TTP, supported by retrospective data showing 80% remission.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from December 2025.

With the priority review, the Prescription Drug User Fee Act date is set for April 29, 2026, for potential approval in this younger indication.

The FDA approved narsoplimab-wuug as the first targeted treatment for transplant-associated thrombotic microangiopathy in adults and children.

Take a look back at the top 5 FDA approvals of 2025.

Approval of the expanded indication fullfilled all post-marketing requirements outlined in the original approval letter.

Ferric maltol is now an FDA-approved oral iron therapy for both adults and adolescents with iron deficiency, aged 10 years and older.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Once-weekly navepegritide improved growth velocity, skeletal alignment, and functional outcomes in children with achondroplasia, with a favorable safety profile and potential implications for long-term care.

The FDA approved fibrinogen, human-chmt to treat acute bleeding episodes in adult and pediatric patients with congenital fibrinogen deficiency.

Abbott received FDA clearance for a new delivery system designed to simplify PDA closure in premature infants weighing as little as 2 lb.

Phase 3 data show the Viaskin peanut patch improved desensitization vs placebo in children aged 4 to 7 years, supporting a planned FDA BLA in 2026.