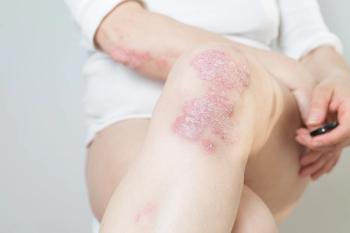
Arcutis Biotherapeutics, Inc has submitted a supplemental New Drug Application for roflumilast cream (ZORYVE) to the US Food and Drug Administration for treatment of plaque psoriasis in children aged 2 to 11 years.

Arcutis Biotherapeutics, Inc has submitted a supplemental New Drug Application for roflumilast cream (ZORYVE) to the US Food and Drug Administration for treatment of plaque psoriasis in children aged 2 to 11 years.

If approved, according to AbbVie, linaclotide would be the first prescription therapy for FC in this patient population.

The FDA has approved atezolizumab for use in patients aged 2 years and older with unresectable or metastatic alveolar soft part sarcoma.

Boehringer Ingelheim and Eli Lilly have announced positive results from a phase 3 trial for Jardiance in reducing blood sugar levels in children with type 2 diabetes.

The US FDA announced the approval of teplizumab (Tzield), which is administered through IV infusion once daily for 14 consecutive days, for delaying the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.
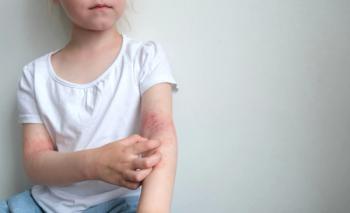
Recently announced data from the INTEGUMENT-1 phase 3 clinical trial demonstrated positive safety and efficacy results for atopic dermatitis patients 6 years and older treated with roflumilast cream (Arcutis Biotherapeutics).

At ACR Convergence 2022, Amit Golding, MD, PhD, outlined drugs recently approved by the FDA, and discussed the requirements for biosimilars to meet approval.

At ACR Convergence 2022, Hyun Son, PharmD, discussed the role of the FDA’s Drug Shortage Staff in preventing and mitigating drug shortages.

Tina Q. Tan, MD, FAAP, FIDSA, FPIDS, sits down to discuss the rising threat of a "triple epidemic" consisting of influenza, COVID-19, and RSV in children.

Elia Pestana Knight, MD; and Whitney Mitchell discuss how CDKL5 deficiency disorder affects children, and how ganaxolone (ZTALMY; Marinus) can drastically improve and even save lives.
Pfizer announced positive data from a phase 3 trial on a bivalent RSV vaccine.

The FDA recently approved a drug treatment for HBV infection for pediatric patients 12 and older with compensated liver disease.
An advisory panel for the US Food and Drug Administration voted 14-1 to withdraw 17α-hydroxyprogesterone caproate (Makena; Covis Pharma) from the market.
Dr. Hyams, MD, discusses functional constipation in children and how positive results from a phase 3 trial on linaclotide will change treatment.

Moderna has announced that their latest BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine, mRNA-1273.222, has been granted Emergency Use Authorization by the US Food and Drug Administration.

Eagle Pharmaceuticals and Enalare Therapeutics have announced that their ENA-001 drug for the treatment of Apnea of Prematurity has been granted an Orphan Drug Designation by the US Food and Drug Administration.
The US Food & Drug Administration approved tetanus toxoid, reduced diptheria toxoid and acellular pertussis vaccine, adsorbed [Tdap] (Boostrix, GSK) today for immunization during the third trimester of pregnancy to prevent pertussis—or whooping cough—in infants under 2 months of age.

The approval, awarded to Alnylam Pharmaceuticals, is based on the results of the ILLUMINATE-C phase 3 trial.

Pfizer and BioNTech have applied to the FDA for Emergency Use Authorization of their Omicron BA.4/BA.5-adapted bivalent vaccine booster for use in pediatric patients aged 3 to 11 years.

AVROBIO’s AVR-RD-04 therapy has been granted rare pediatric disease designation to treat cystinosis in pediatric patients.
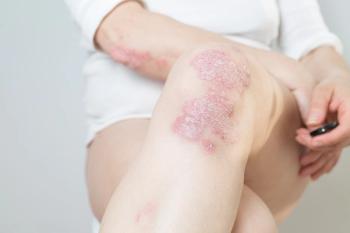
In 2 recent phase 3 trials, safety and efficacy was proven in the use of roflumilast cream 0.3% for the treatment of plaque psoriasis.

Sodium thiosulfate has been approved by the FDA to treat ototoxicity in pediatric patients with localized, non-metastatic solid tumors.

After promising outcomes in an ongoing phase 2 trial, the FDA has given Pfizer’s GBS6 vaccine Breakthrough Therapy Designation against Group B Streptococcus in infants.

Bluebird bio has announced that their SKYSONA therapy has been approved for pediatric use by the FDA.

Aro Biotherapeutics’ ABX1100, used to treat Pompe disease, was granted a Rare Pediatric Drug designation by the FDA.