
Bivalent COVID-19 vaccines by both Moderna and Pfizer-BioNTech are no longer authorized for use in the United States.

Bivalent COVID-19 vaccines by both Moderna and Pfizer-BioNTech are no longer authorized for use in the United States.

After this summer’s Canadian wildfires, should we be concerned about future air quality emergencies? This article was originally published on our sister publication’s website, Drug Topics®.
Monovalent vaccines by these manufacturers are no longer authorized for use in the United States.

Stay on the nice list with these tips to help stop the spread of winter respiratory viruses.
The decision comes following reports of 16,000 cases across 75 countries and territories around the world.

The approval represents an adjunctive therapy option for pediatric patients older than age 16, as well as adults.
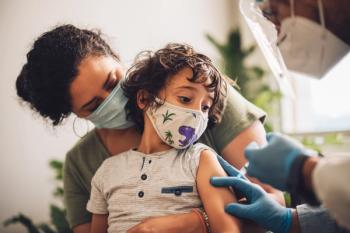
Children may receive a single booster shot at least 5 months after completing their initial Pfizer-BioNTech 2-dose series.

Asthma may increase patient risk of developing dry eye disease.
The US Food and Drug Administration (FDA) is considering dispensing mail-back envelopes with outpatient opioid analgesic prescriptions.
Multiple pharmaceutical companies are investigating the application of mRNA technology to develop vaccines that treat and prevent multiple conditions, from influenza to cancer and beyond.
The NVX-CoV2373 vaccine was evaluated in adolescents aged 12 to 17 years.
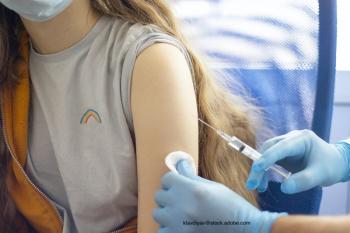
The US Food and Drug Administration (FDA) has extended the emergency use authorization for the Pfizer/BioNTech COVID-19 vaccine to include a booster dose for teenagers aged 16 to 17 years.

Published: May 10th 2022 | Updated: