
Expanding peripartum prophylaxis could be more cost-effective, but would likely lead to significant over-treatment.


Expanding peripartum prophylaxis could be more cost-effective, but would likely lead to significant over-treatment.

Investigators studied 2 infants experiencing seizures, small head size, and developmental delays born of mothers who had contracted COVID-19 during pregnancy.

These findings suggest the World Health Organization's definition of post-COVID-19 condition (long COVID) may be too broad.

Children exposed to maternal COVID-19 during pregnancy showed lower birth weight, lower birth BMI, and accelerated postnatal weight gain, compared with those unexposed.
In a recent study, female offspring of mothers with a positive COVID-19 test during pregnancy were at a decreased risk of developing neurodevelopmental disorders compared to male offspring.
New maternal vaccine options could offer extra protection in upcoming seasons.

Exposure to a common seasonal coronavirus stimulates a memory T cell response that protects children against COVID-19. However, this immune response peaks at age 6.
If approved by the FDA, this 5-in-1 investigational candidate could provide the broadest meningococcal serogroup coverage.
At the 44th National Association of Pediatric Nurse Practitioners Conference, data was presented on the effectiveness of vaccines and antivirals in preventing life threatening conditions.
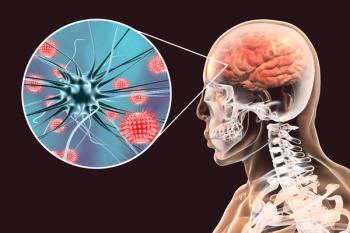
Assessing your knowledge of and performance on preventable diseases.
The Omicron variant caused the most symptoms in pediatric patients. However, there were no differences in adverse outcomes by COVID-19 variant.
Children were hospitalized the most during the Omicron variant, but disease outcomes were the least severe during this variant period.

The federal agency set a Prescription Drug User Fee Act (PDUFA) action date of August 2023.

In a recent analysis, worse maternal and neonatal outcomes were found when mothers were infected with COVID-19 close to delivery.

A recent grant awarded to Ann & Robert H. Lurie Children's Hospital of Chicago will look at the severity score of emergency department (ED)-based pediatric community-acquired pneumonia.

To address the increased demand for fever-reducing medications, the FDA has updated its compounding guidelines for licensed pharmacies, hospitals, and health systems.
In a recent study, children aged under 5 years with COVID-19 more often presented with severe illness when comorbid with another respiratory illness.
More contagious but less severe, XBB takes over as dominant Omicron strain of COVID-19

When analyzing morbidity data from 2021, investigators found that COVID-19 was among the 10 leading causes of death among children and young people.

Worst of ‘tripledemic’ may be over.

New maternal vaccine options could offer extra protection in upcoming seasons.

The US Food Administration has agreed to review an application for nirsevimab (Beyfortus; AstraZeneca and Sanofi) to protect infants against RSV.

The results show similar rates o colonized C difficile among pediatric patients and adult patients with cystic fibrosis.

Tina Q. Tan, MD, FAAP, FIDSA, FPIDS, editor-in-chief of Contemporary Pediatrics, talks about the most significant moments in children’s health in 2022; what the tridemic really means for pediatric health care providers; and what we have to look forward to in 2023 when it comes to new vaccines and medications.

Alok Patel, MD, shares what pediatric health care providers should know about a concerning new strain of group A streptococcus.