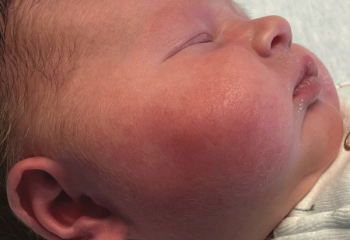
An 18-day-old girl whose right cheek had become increasingly red and warm over 24 hours was directly admitted to an inpatient unit. She had firmness and pain to the affected area, fussiness, increased sleeping, and poor feeding, preferring the bottle to breastfeeding. What's the diagnosis?