
Latest emergency use authorizations for Moderna, Pfizer-BioNTech vaccines.

Latest emergency use authorizations for Moderna, Pfizer-BioNTech vaccines.
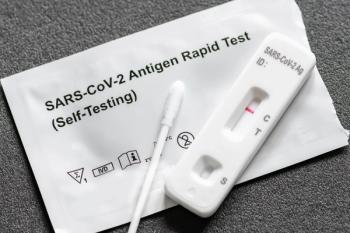
In a recent study, children were successfully able to self-swab and receive their own COVID-19 test results after basic instructions.

Craig Shapiro, MD, pediatric infectious disease specialist at Nemours Children's Hospital, sits down to discuss the recent uptick in pediatric monkeypox cases and explains what to do if you suspect your patient to have monkeypox.

Novavax joins the growing list of COVID-19 vaccines including vaccines from Pfizer-BioNTech, Moderna, and Johnson & Johnson’s Janssen

The news is full of stories about monkeypox, the recent changes to COVID-19 guidance, and the return of polio cases. What do clinicians need to know?

A 29-year-old White woman presented to the labor and delivery unit due to preterm premature rupture of membranes and delivered twins. The twins were transferred to the neonatal intensive care unit following delivery.

The American Academy of Pediatrics has outlined safety tips to keep children physically and emotionally healthy as they return to school this season.
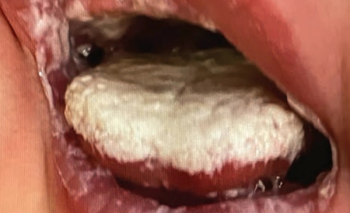
A 4-week-old boy presents with creamy white patches on his lips, right and left buccal mucosa, palate, and tongue.
The FDA has granted Emergency Use Authorization (EUA) of the JYNNEOS vaccine for pediatric patients <18 years who are at high risk of monkeypox infection.
Only one-fifth of parents in a recent survey indicated intentions of getting their child the vaccine within 3 months of eligibility.
White House ramps up efforts to fight outbreak.
Learn about the selected strains for this year’s flu season, flu vaccine recommendation changes, and newly licensed and forthcoming vaccine options.
As immunization rates fall in children, the American Academy of Pediatrics recommends a checkup with physicians.

Many students are returning to a regular schedule for the first time since 2020. How will they fare?

The American Academy of Pediatrics (AAP) recommends children aged 5 to 11 receive the BNT-162b2 vaccination.

Codes enable billing for lab tests, administering vaccines.

The decision comes following reports of 16,000 cases across 75 countries and territories around the world.
Purchase follows FDA recommendation to include Omicron component in boosters.

The pandemic has disrupted academic progress and children with learning disabilities in particular have been impacted. Here is what pediatricians should know to help support literacy and recognize dyslexia.

Verrica is complying with the FDA through 3 Complete Response Letters to achieve the first FDA-approved treatment of molluscum contagiosum.

In children 5-11 years old, 2 doses of the Pfizer-BioNTech COVID-19 vaccine were moderately effective against documented, symptomatic Omicron infection.
Patients with a history of infection who were also vaccinated had the strongest protection, the data showed.
Recently, the AAP announced the launch of a public health campaign to educate caregivers on the benefits of vaccinating all ages.

The stress of enduring an infectious disease pandemic expresses itself in a variety of mental health conditions, but the likelihood of experiencing particular symptoms depends on who you are.

Data from Morbidity and Mortality Weekly Report showed that there was no demonstrated increase in pediatric hepatitis or adenovirus 40/41 above pre-COVID-19 pandemic baseline levels.