Endocrinology
Latest News
Latest Videos

CME Content
More News

The primary outcome was an elevated HbA1c level greater than or equal to 5.7% (prediabetes or undiagnosed presumed T2D).

Drugs like semaglutide and liraglutide were dispensed at unprecedented increased rates for adolescents and young adults over a span of just 4 years.

Investigators found that higher baseline HbA1c was associated with greater T2D risk.

The FDA has approved lutetium Lu 177 dotatate for pediatric patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors.

Lara Smith, DNP, APRN, CPNP-AC/PC, provides case-based examples of endocrine emergencies in the pediatric ICU, based on her session presented at the 45th NAPNAP National Conference.
A qualitative analysis shows adolescents and their parents more frequently report an unreadiness to transition from pediatric to adult type 1 diabetes care.

Crinecerfont, an investigational, oral, selective corticotropin-releasing factory type 1 receptor antagonist, achieved the primary and key secondary endpoints in a phase 3 study to treat congenital adrenal hyperplasia due to 21-hydroxylase deficiency in children aged 2 to 17 years.

According to the study, maintaining modest residual beta cell function in newly diagnosed patients is associated with a lower risk of vascular complications and hypoglycemia.

The US FDA announced the approval of teplizumab (Tzield), which is administered through IV infusion once daily for 14 consecutive days, for delaying the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.


An analysis of data from more than 9900 people with Down syndrome and 38,000 controls provides new insight into the apparent increase in risk of developing diabetes among children and young adults with Down syndrome.

Results of the TARGET trial provide insight into the effects of tight glycemic targets versus less tight targets on maternal and perinatal outcomes among women with gestational diabetes.

Investigators found minimal difference between breastfeeding rates less than 1 week postpartum of women who had diabetes pre-pregnancy, gestational diabetes only, and no history of diabetes.

New research suggests adolescent obesity could increase risk of type 1 diabetes in young adulthood.
An analysis of the SEARCH for Youth in Diabetes details historic and contemporary disparities in insulin pump use for pediatric type 1 diabetes based on racial/ethnic background, household income, and insurance type.

An analysis of more than 100 mother-child pairs from Colorado found children with fetal exposure to cannabis had increased fat mass and fasting glucose levels compared to their counterparts without fetal exposure to cannabis.

Puberty will happen for every child, but when it arrives early or is delayed both parent and child may be worried. A presentation at the virtual 2021 American Academy of Pediatrics National Conference & Exhibition discussed typical reasons for both early and delayed pubertal development.
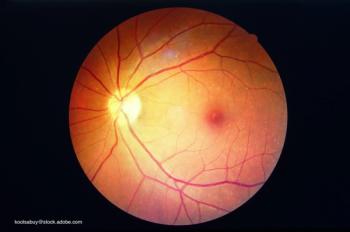
With both type 1 and type 2 diabetes on the rise in children, it’s important to understand the factors that can lead to the development of diabetic retinopathy.

The US Food and Drug Administration (FDA) has approved the AstraZeneca once-weekly injectable therapy for use in children aged 10 to 17 years.
A session at the virtual Scientific Sessions for the American Diabetes Association offered some best practices for the diagnosis and treatment of hypoglycemia.
A session at the virtual Scientific Sessions for the American Diabetes Association examined the potential long-term outcomes for a child who experiences neonatal hypoglycemia.

A study presented during a poster session at the American Diabetes Association Virtual 81st Scientific Sessions evaluated performance, safety, and satisfaction of an extended-wear insulin infusion set in patients with type 1 diabetes.

A session at the virtual Scientific Sessions for the American Diabetes Association examines the role food insecurity may play in glycemic control and acute complication among type 2 diabetes patients.

The impact of nutrition in the first years of life on future cardiometabolic health has been generally understood, although gaps about certain populations remain. A session at the virtual Scientific Sessions for the American Diabetes Association sought to fill the gap for Native American children.