The influenza vaccine has been recommended as a key way to reduce severe influenza in children. An investigation examines how effective the 2018-2019 vaccine was in preventing hospitalization and emergency department visits.

The influenza vaccine has been recommended as a key way to reduce severe influenza in children. An investigation examines how effective the 2018-2019 vaccine was in preventing hospitalization and emergency department visits.
The 2020-2021 influenza season will be a strange one because of COVID-19. The National Foundation for Infectious Disease (NFID) aired a webcast that addressed how important the influenza vaccine was going to be to keep people safe during uncertain times.
Human papillomavirus (HPV) vaccination coverage has lagged in the United States. A report looks at whether the coverage will meet the Healthy People 2020 goal.
Dr. Tina Tan addresses the recent change that allows pharmacists to administer vaccinations to pediatric patients.
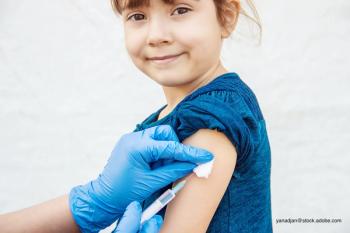
The 2020-2021 influenza season is fast approaching and the American Academy of Pediatrics (AAP) has issued their annual recommendations.
Contemporary Pediatrics sat down with Andrew J. Schuman, MD, to discuss vaccine hesitancy and how those sentiments could impact the future COVID-19 vaccine.
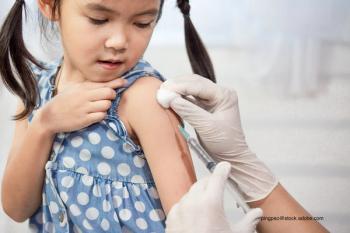
This is the critical moment in time for all health care providers to proactively stop this potential public health nightmare by speaking with all parents and adolescents about the absolute need for everyone to receive the influenza vaccine.

The US government has entered into an agreement to acquire the first 100 doses of a COVID-19 vaccine candidate developed by Moderna, Inc., pending approval by the US Food and Drug Administration.

As the COVID-19 pandemic continues, a new question arises: should schools with in-person classes require students and their family to get the flu vaccine?

A study looks at whether a vaccine for respiratory syncytial virus (RSV) could be effective in reducing RSV-associated, medically significant lower respiratory tract infections in infants.
Three scientists discuss the global race for a COVID-19 vaccine.
A program with the goal of expanding human papillomavirus (HPV) vaccination and preventing HPV-related cancers has been expanded.

A recent study offers hope for more effective vaccine responses.
Immunizations during pregnancy are common and meant to provide protection during the early months of life. A literature review looks at whether the practice influences other early health outcomes.

The US government has entered into an agreement to acquire the first 100 doses of a COVID-19 vaccine candidate developed by Pfizer and BioNTech, pending approval by the US Food and Drug Administration.

Routine vaccination for meningococcal disease has been recommended since 2005. A study looks at whether the recommendation has reduced the incidence of disease.

Walgreens announces that routine immunization service will resume.
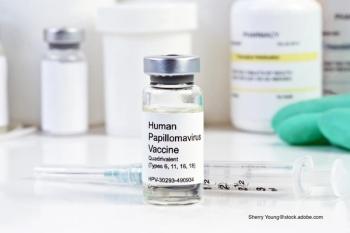
Initiation and completion rates for human papillomavirus (HPV) vaccine aren’t where public health officials would like them to be. A report examines whether an intensive intervention can help improve those rates.

Vaccine hesitancy is a major issue in pediatrics and many have wondered how prevalent the issue is. A new survey offers a dispiriting answer.

New research shows that a mother’s mental illness can impact their child’s completion of recommended vaccinations.

Standing orders have a positive impact on vaccination rates. However, a new study indicates that some doctors aren’t using them for a variety of reasons.

Autodialer centralized reminder and recall messages have been used to improve the rate of childhood vaccination. A new study examines whether they could improve human papillomavirus (HPV) vaccination rates as well.

COVID-19 has led to many changes in health care, including how children receive care. A new report from the Centers for Disease Control and Prevention examines how these changes have impacted vaccination.

Current US vaccine policy recommends that children receive 2 influenza vaccine doses during their first influenza season. A new study indicates that this helps reduce influenza burden among the vulnerable population.
The US FDA has approved Sanofi’s meningococcal (groups A, C, Y, W) conjugate vaccine (MenQuadfi) to prevent invasive meningococcal disease in children aged 2 years or older.