
Contemporary Pediatrics sat down with editor-in-chief Dr. Tina Q. Tan to discuss the authorization of the Pfizer/BioNTech vaccine for use in many children as well as what lies ahead in the vaccine pipeline for the pediatric population.

Contemporary Pediatrics sat down with editor-in-chief Dr. Tina Q. Tan to discuss the authorization of the Pfizer/BioNTech vaccine for use in many children as well as what lies ahead in the vaccine pipeline for the pediatric population.
Paul Offit, MD, discusses the state of COVID-19 vaccines and children.
A look at the Centers for Disease Control and Prevention's (CDC) vaccination schedule for the pediatric population.

Not every vaccine is required, some are just strongly recommended. Here's what to know about 5 recommended vaccines.

A look at where vaccine technologies will be going.

Contemporary Pediatrics sat down with editor-in-chief Dr. Tina Q. Tan to discuss the what lies ahead in the vaccine pipeline for the pediatric population.
What we can look forward to in preventing infectious diseases.
A study examines whether an intervention that promotes including teenagers in the decision-making process for getting vaccines leads to better outcomes, such as vaccine literacy.
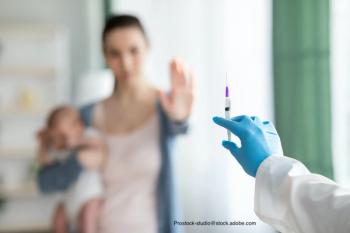
Best tactics for pediatricians when speaking with patients who resist immunization.

A look at vaccine registries: what they are, and why they are so crucial.

Sharing how vaccinations first began and were implemented can be a useful tool in educating families of their importance.
Ocugen, Inc. has submitted a request to the US Food and Drug Administration for emergency use authorization (EUA) for their COVID-19 vaccine candidate for use in children aged 2-18 years.
Effective vaccines are one of the greatest public health achievements. Dr. Tina Q. Tan shares how the November issue is dedicated to vaccines, from the history to tackling vaccine hesitancy.
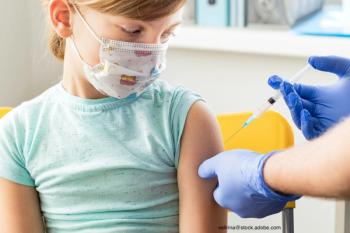
Contemporary Pediatrics sat down with editor-in-chief Dr. Tina Q. Tan to discuss the approval of the COVID-19 vaccine for children aged 5 to 11 years and conversations with parents.

The US Food and Drug Administration (FDA) has amended the emergency use authorization of the Pfizer/BioNTech vaccine to include children aged 5 to 11 years.

The US Food and Drug Administration (FDA) committee voted on whether the emergency use authorization should be extended to children aged 5 to 11 years.

The US Food and Drug Administration (FDA) is meeting to discuss amending the emergency use authorization of the Pfizer/BioNTech vaccine for children aged 5 to 11 years.

Seqirus’s FLUCELVAX® cell-based quadrivalent influenza vaccine has been approved to expand the age indication to children as young as 6 months of age by the US Food and Drug Administration.

Contemporary Pediatrics sits down with Dr. Donna Hallas, a clinical professor at the New York University Meyers College of Nursing and also a pediatric nurse practitioner, about the upcoming flu season and keeping families safe.
Initial results from the trial indicate it was well-tolerated and promoted a good immune response.

Anthony S. Fauci, MD, Director of the National Institute of Allergy and Infectious Disease, reveals when he believes a COVID-19 vaccine for children aged less than 12 years will be available.

The American Academy of Pediatrics (AAP) has issued its recommendations for the upcoming influenza season, including guidance on administering the vaccine to patients with COVID-19.

Although the vaccine rollout has snagged, the effort has already saved thousands.
Merck has released promising topline results from their study of VAXNEUVANCE™, a 15-valent pneumococcal conjugate vaccine, in infants.

Faced with the fourth highest vaccine exemption rate, Michigan began requiring an education session before a parent could get a nonmedical exemption. Did it lead to an improvement?