
Patients with a history of multisystem inflammatory syndrome in children (MIS-C) completed a questionnaire about adverse reactions following COVID-19 vaccination, and no serious adverse events were recorded.

Patients with a history of multisystem inflammatory syndrome in children (MIS-C) completed a questionnaire about adverse reactions following COVID-19 vaccination, and no serious adverse events were recorded.
In a recent report, the Centers for Disease Control and Prevention reported that the JYNNEOS vaccine for preventing monkeypox has not caused severe adverse events in patients aged under 18 years.
A recent analysis showed that vaccine-associated myopericarditis was rare in adolescents and young adults, and that outcomes were favorable.

The US Food and Drug Administration has updated the emergency use authorization for bivalent COVID-19 vaccines to be available for children aged 6 months and older.
In a recent study, infants born from mothers vaccinated against influenza were less likely to be hospitalized from the disease than those born to unvaccinated mothers.

COVID-19 has caused millions of children worldwide to miss their measles vaccinations, putting them at increased risk of measles infection.
Bradley Warady, MD, pediatric nephrologist and researcher at Children’s Mercy Kansas City, discusses the causes of vaccine hesitancy and the effects of vaccine hesitancy on public health.
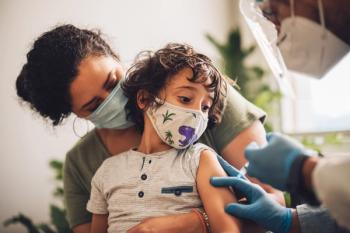
A recent poll showed that some parents do not discuss vaccines with their child’s regular doctor, with many choosing to not have their child receive any vaccines.

A real-world study determined the efficacy of mRNA vaccines for protection against COVID-19 in adolescents.
Confidence in vaccines is lower post-pandemic across all demographic groups.
Despite vaccine effectiveness against human papillomavirus, many individuals do not receive vaccination prior to sexual debut, as recommended by the ACIP.
Donna Hallas, CPNP, FAAN, FAANP, PMHS, PPCNP-BC, PhD, discusses the top news from the October issue of Contemporary Pediatrics®.

This article discusses past and future development of mRNA vaccines, and how they have been used throughout the COVID-19 pandemic.
Deborah Molrine, MD, Clinical Program Director, QIVc at CSL Seqirus, discusses CSL Seqirus’ cell-based quadrivalent seasonal influenza vaccine, along with the effectiveness of cell-based vaccines in children.

As COVID-19 continues to spread, vaccinations remain available to protect children from severe disease, hospitalization, and death.

This article discusses the process of vaccine development, and how health care personnel can confidently discuss the subject with parents and patients.

Moderna has announced that their latest BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine, mRNA-1273.222, has been granted Emergency Use Authorization by the US Food and Drug Administration.
In this article, declining trends in vaccination across the United States are discussed, along with how providers can reduce vaccine hesitancy.
Study links discrepancy to adults’ misbeliefs about vaccine safety.

Pfizer and BioNTech have applied to the FDA for Emergency Use Authorization of their Omicron BA.4/BA.5-adapted bivalent vaccine booster for use in pediatric patients aged 3 to 11 years.

In a recent report, the American Academy of Pediatrics discussed how influenza affects children and how it can be prevented.
In a recent study, participants who had received a COVID-19 vaccine saw an average increase in menstrual cycle length of less than 1 day.
In a new statement, the American Academy of Pediatrics outlines the importance of immunization information systems.
In 2 new education documents, Physicians for Informed Consent outlines the risks of Hepatitis B compared to the risks of the Hepatitis B vaccination.
Infants of fully vaccinated mothers had fewer cases of hospitalization from COVID-19 than those unvaccinated.