
Patient portals can be a powerful communication tool for both clinician and family, but can they also be used to improve uptake of the annual influenza vaccine?

Patient portals can be a powerful communication tool for both clinician and family, but can they also be used to improve uptake of the annual influenza vaccine?
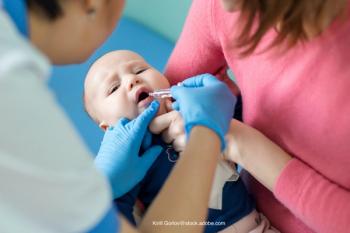
Rotavirus can lead to gastrointestinal concerns that can be hazardous to children when rehydration isn’t readily available, which means that a safe, effective vaccine could be life-saving. A meta-analysis examines the safety and efficacy of available immunizations.
Educating families to alleviate fear in pediatric populations regarding the flu vaccine will improve the vaccination rate and protect high-risk children. Due to the negative effects of the flu in children with diabetes, it is vital that at-risk youth be vaccinated.

Contemporary Pediatrics speaks with Dr. Jeffrey Gerber about when we will see COVID-19 vaccines for children aged less than 12 years.

There are many reasons why a caregiver may refuse to have his or her child receive the human papillomavirus (HPV) vaccine, including safety concerns. A research letter examines whether those concerns have increased.
The last meeting of the COVID-19 vaccine congress from the International Society for Vaccines (ISV) provided information on the hypersensitivity events tied to mRNA vaccines as well as the most recent information on the Janssen and Novavax vaccines.

The company has publicized data from the TeenCOVE study of the vaccine along with plans to seek authorization in the coming weeks.
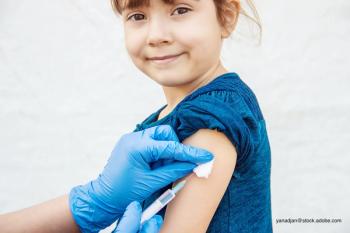
Contemporary Pediatrics sat down with Dr. Donna Hallas to discuss how to communicate with parents as well as how to address vaccines missed because of the pandemic.
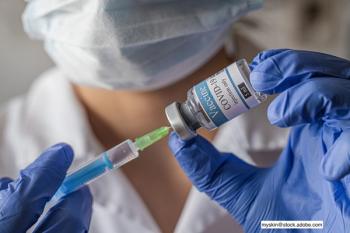
The American Academy of Pediatrics (AAP) issued a statement that urges parents get all children and teenagers aged 12 and older vaccinated with Pfizer/BioNTech vaccine, which recently had its authorization expanded.

The US Food and Drug Administration expanded the emergency use authorization of the 2-dose vaccine to include children aged 12 to 15 years.
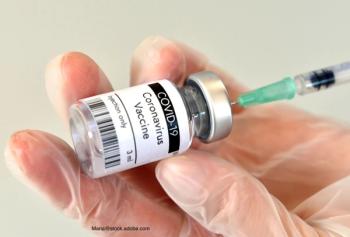
The International Society for Vaccines (ISV) presented its fifth update on COVID-19 vaccines around the world. This update focused on vaccines produced in China.
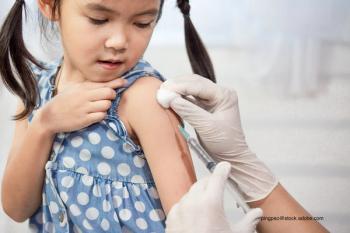
Tackling vaccine hesitancy for the coronavirus disease 2019 (COVID-19) is going to be an important component of achieving the herd immunity needed to end the pandemic.
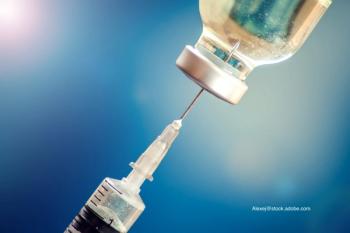
Pfizer/BioNTech has released data on their coronavirus disease 2019 (COVID-19) vaccine that indicates that its highly effective in children aged 12 to 15 years.

Moderna announces beginning of coronavirus disease 2019 (COVID-19) vaccine trial that include children aged 6 months to >12 years.
A safe coronavirus 2019 disease (COVID-19) vaccine that can be administered to most of the pediatric population is a necessity for ending the pandemic, but trials for younger children have not occurred. The president of the American Academy of Pediatrics (AAP) wrote to key COVID-19 officials about the need to change this.
As the 2020-2021 influenza season loomed, many in health care worried that the seasonal disease would add to the extraordinary burden of coronavirus disease 2019 and create a perfect storm. Have various prevention strategies helped prevent this potential issue?

Vaccine hesitancy is nothing new in pediatrics. However, the increased hesitancy for the COVID-19 vaccine could prove problematic.

Improving influenza vaccination coverage is important for all children, but some are at greater risk for poorer outcomes. A review examines whether interventions can improve vaccine coverage.
A report examines if the influenza and pneumococcal vaccine has a protective effect against COVID-19.

The American Academy of Pediatrics (AAP) has issued initial guidance for COVID-19 vaccination in pediatrics.

Moderna has announced further research into whether a booster shot could provide additional protection with new COVID-19 variants.

The COVID-19 vaccine, and the speed at which it was developed, is the medical breakthrough of our lifetimes.

Social distancing measures may have helped slow the spread of COVID-19 in the early days of the pandemic, but an investigation indicates an unintended and potentially problematic consequence: a decline in vaccination.
Following the green light from the vaccine advisory committee, the US Food and Drug Administration has given emergency use authorization for the Pfizer/BioNTech COVID-19 vaccine, making it the first vaccine for COVID-19 approved for use in the United States.

The US Food and Drug Administration (FDA) vaccine committee has given the green light to the the Pfizer/BioNTech vaccine, meaning approval may happen in a matter of days.