A minimum expiratory tidal volume of 4 mL/kg is associated with successful lung aeration during delivery room resuscitation of preterm neonates.

Joshua Fitch is the senior editor for Contemporary Pediatrics. He joined the brand in March of 2023 as an editor before being promoted to senior editor in January 2024. Fitch graduated from Youngstown State University in Youngstown, Ohio in 2020 with a degree in telecommunications and journalism. He started his career as a news and sports videographer before becoming an on-air sports anchor at the NBC-affiliated news station in Youngstown. Fitch briefly worked as a national content writer for a Chicago-based national television station before joining the Contemporary Pediatrics team. He can be reached at: jfitch@mjhlifesciences.com.

A minimum expiratory tidal volume of 4 mL/kg is associated with successful lung aeration during delivery room resuscitation of preterm neonates.

Brensocatib was granted FDA priority review in February 2025 for the small molecule, oral, reversible inhibitor.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
Findings may help guide clinicians and policymakers in balancing the risks and benefits of stimulant prescribing in adolescent populations, according to the study authors.

Pediatric experts emphasize the importance of asthma action plans, medication readiness, and school-based support to help children with asthma stay healthy and active during the academic year.

The expanded label of tocilizumab-anoh now includes treatment for patients aged 2 years and older with cytokine release syndrome (CRS).

The indication approved is for children aged 6 years and older who weigh at least 45 kg (99 lbs) for the preventive treatment of episodic migraine.

Though intensive nurse home services did not change outcomes in this South Carolina study, authors noted more research is needed on a larger scale to determine influence on adverse outcomes.

As families prepare for a new school year, pediatricians are essential in helping parents navigate immunization schedules and school requirements and ensuring vaccine confidence.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
New CDC data show fewer kindergartners are fully vaccinated as exemption rates increased during the 2024-2025 school year.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from July 2025.

Take a quick look at everything you may have missed last month, including the top FDA approvals and latest clinical updates.
“While our study is small, its results are powerful and have implications not only for MIS-C, but potentially for long COVID,” said lead author Lael Yonker, MD.

Achieving the primary end point, 44.6% and 54.3% of patients treated with upadacitinib (15 mg and 30 mg) reached 80% or more scalp hair coverage.

Nektar Therapeutics remains on track to announce top-line phase 2 data in December.

Pegcetacoplan (Empaveli; Apellis Pharmaceuticals) is indicated for patients 12 years or older to reduce proteinuria.

“Without treatment, individuals with PKU would have very high blood Phe levels...so that results in neurocognitive deficits and mood disorders," Suzanne Hollander, MS, RD, LDN, said.
The study enrolled infants not selected for risk, a population that has been seldom studied with regard to emollient intervention.
Of the 253 influenza-related pediatric deaths through June 21, 2025, 42.7% occurred in children without a high-risk medical condition.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Study links early childhood eczema timing to risk of allergies like food allergy, asthma, and rhinitis, with five distinct disease phenotypes identified.

This week, HHS Secretary Robert F. Kennedy Jr signed the ACIP's recommendation to remove the mercury-based preservative from all influenza vaccines.

Solid Biosciences is planning a phase 1b clinical trial of SGT-501 to treat the catecholaminergic polymorphic ventricular tachycardia heart condition.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

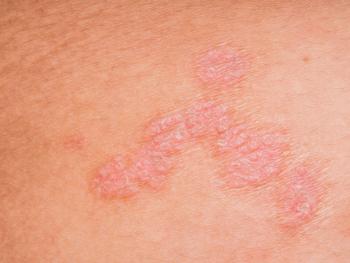
J&J is seeking the first approval for the investigational targeted oral peptide to treat patients 12 years or older with moderate to severe plaque psoriasis.

A new study aimed to determine mental health resilience in preterm-born children and identify modifiable factors associated with resilience.

According to research from 2021 to 2023, approximately 50% of adolescents who vaped had tried to quit in the last 12 months.

The nitric oxide–releasing topical is the first prescription medication approved for at-home treatment of molluscum contagiosum.
A newly-published study identified specific groups of higher-risk children that could benefit most from monoclonal antibodies.