
Submission is based on 3 clinical trials, including data from the ApproaCH Trial among children with achondroplasia.

Joshua Fitch is the senior editor for Contemporary Pediatrics. He joined the brand in March of 2023 as an editor before being promoted to senior editor in January 2024. Fitch graduated from Youngstown State University in Youngstown, Ohio in 2020 with a degree in telecommunications and journalism. He started his career as a news and sports videographer before becoming an on-air sports anchor at the NBC-affiliated news station in Youngstown. Fitch briefly worked as a national content writer for a Chicago-based national television station before joining the Contemporary Pediatrics team. He can be reached at: jfitch@mjhlifesciences.com.

Submission is based on 3 clinical trials, including data from the ApproaCH Trial among children with achondroplasia.

Jay T. Rubinstein, MD, PhD, emphasized that children with hearing loss should receive genetic testing, as the investigational DB-OTO gene therapy demonstrated positive results.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Fitusiran is administered subcutaneously starting once every 2 months for patients aged 12 years and older with hemophilia A or hemophilia B, with or without factor VIII or IX inhibitors.

A look ahead to 5 key regulatory decisions scheduled to take place during the second quarter of 2025, from a monoclonal antibody to topical psoriasis treatments.
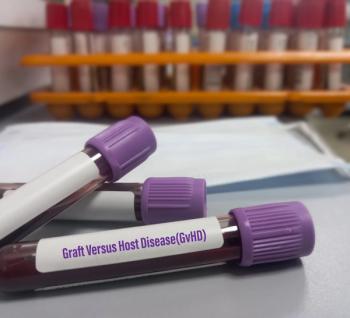
Mesoblast's remestemcel-L (Ryoncil) was approved by the FDA on December 18, 2024 to treat SR-aGVHD in patients 2 months and older. Now, it is available for purchase.

Peter Lio, MD, offers thoughts on recent atopic dermatitis data reported for lebrikizumab.

Diazoxide choline has been granted breakthrough, fast track, and orphan drug designations in the United States.

In this Q+A interview, Craig McDonald, MD, discusses the interim, 90-day data from the phase 1/2 INSPIRE DUCHENNE trial, evaluating SGT-003, a gene therapy candidate for DMD.

Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
Pediatric cancer survivors can be vulnerable to cardiovascular disease in the short- or long-term with increased recognition of cardiotoxic cancer treatments, as 5-year survival rates for children are greater than 85%.

Walker and Harris emphasize the importance of early hearing loss detection, screening, and intervention to support optimal child development.
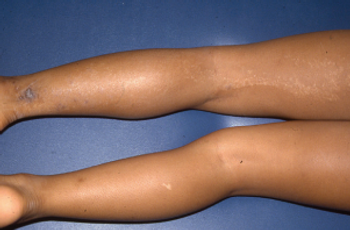
Take your best guess and try to diagnose this 9-year-old girl's linear eruption on the back of her left thigh and leg. Stay tuned for the full case presentation and correct diagnosis!

Peter S. Jensen, MD, discusses the risks and benefits of social media use by children, emphasizing the need for parental oversight and national guidelines.

Results were from the phase 3 STEER study among a broad population of patients with SMA aged 2 to under 18 years.

The federal agencies are encouraging companies to develop new infant formulas and clarify opportunities to inform consumers about formula ingredients.

Danielle Van Damme, DNP, CPNP-AC, discussed key updates from the 2024 IDSA guideline on diagnosing complicated intra-abdominal infections, emphasizing ultrasound as the preferred imaging modality.

Prior to approval on February 14, 2025, the only FDA approved vaccine for chikungunya protection was indicated for 18 years and up.

Karen Y. Capusan, DNP, CPNP-PC, explored RSV’s shifting epidemiology, seasonal trends, and new vaccines’ role in reducing hospitalizations at the 2025 NAPNAP National Conference.

Pediatricians face rising demands in specialty care and mental health, with programs such as REACH helping them manage these challenges.

At the 2025 NAPNAP Conference in Chicago, Jessica Spruit, DNP, CPNP-AC, FAANP, discussed the AAP's first clinical practice guideline on opioid prescribing.

Bobbie Monaco, MSN, CPNP-PC, provided a recap of her NAPNAP session regarding period poverty, and called for continued advocacy and awareness in the United States and globally.

Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, comments on the ongoing measles outbreaks and encourages advocation for vaccines and accurate information.

Mary Koslap-Petraco, DNP, PPCNP-BC, CPNP, FAANP, highlighted key updates on influenza, COVID-19, and RSV vaccines at the 2025 NAPNAP National Conference.

Tina Tan, MD, FIDSA, FPIDS, FAAP, comments on the FDA's recent selection of the 2025-2026 influenza vaccine compositions, without the CDC's ACIP meeting and FDA's VRBPAC meeting.

Mary Koslap-Petraco highlighted the need to address vaccine hesitancy, catch up on immunizations, and build trust with parents at the 2025 NAPNAP National Conference on Pediatric Health Care.

At the 2025 NAPNAP National Conference, Maureen Madden, DNP, CPNP-AC, CCRN, FCCM, FAAN, explained why the yearly conference is important for both providers and pediatric patients.

Mary Koslap-Petraco, DNP, PPCNP-BC, CPNP, FAANP, reacts to the recent cancelations of the ACIP and VRBPAC and comments on the potential down stream effects.

At the 2025 NAPNAP National Conference, Maureen Madden, DNP, CPNP-AC, CCRN, FCCM, FAAN, provides the key takeaways and recommendations for glycemic control guidance, updated in 2024.