
Tyra Bryant-Stephens, MD, encourages families to revisit asthma triggers, refill medications, and manage allergies to prevent avoidable complications.

Tyra Bryant-Stephens, MD, encourages families to revisit asthma triggers, refill medications, and manage allergies to prevent avoidable complications.

Most youth with type 1 diabetes in sub-Saharan Africa lack autoimmune markers, suggesting a distinct, non-autoimmune form of insulin-deficient diabetes.

This week, HHS Secretary Robert F. Kennedy Jr signed the ACIP's recommendation to remove the mercury-based preservative from all influenza vaccines.


Solid Biosciences is planning a phase 1b clinical trial of SGT-501 to treat the catecholaminergic polymorphic ventricular tachycardia heart condition.
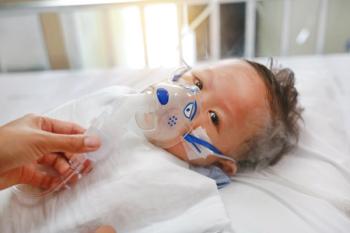
Nirsevimab reduced RSV-related illness by up to 98% and lowered the amount of hospitalizations and health care use among healthy term infants in a real-world study.

Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

John Browning, MD, highlights the importance of fragrance-free, steroid-free creams in managing atopic dermatitis safely and effectively in children.

A new polygenic risk score enhances obesity risk prediction from childhood to adulthood, offering new insights for early intervention and prevention strategies.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

J&J is seeking the first approval for the investigational targeted oral peptide to treat patients 12 years or older with moderate to severe plaque psoriasis.

In this episode of Infectious Insights, our host Tina Tan, MD, is joined by general pediatrician Candice Jones, MD, to discuss an unprecedented vaccine season.

U.S. public schools enhance student mental health support through screenings, yet face challenges in ensuring effective follow-up care and resources.

Lori Handy, MD, MSCE, emphasizes the role of school entry as a critical time for ensuring children are caught up on routine vaccinations.

Pediatric pulmonologist Richard Wong, DO, outlines how school environments can complicate asthma management for children across all age groups.

Discover the latest in pediatric infectious diseases with Infectious Insights, a new podcast series hosted by expert Tina Tan, MD, FIDSA, FPIDS, FAAP.

A new study aimed to determine mental health resilience in preterm-born children and identify modifiable factors associated with resilience.

According to research from 2021 to 2023, approximately 50% of adolescents who vaped had tried to quit in the last 12 months.

Childhood cardiovascular health is crucial for lifelong benefits, enhancing cognitive, metabolic, and mental well-being while reducing disease risks.

Early antibiotic exposure disrupts infant immune development by altering gut microbiota, highlighting inosine's potential as a therapeutic target for immune restoration.

A recent study reveals a staggering 763% rise in nicotine pouch ingestions among young children, highlighting urgent public health concerns.

Parents and pediatricians navigate the complexities of neurodiversity, emphasizing the need for open communication and better resources for child development.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The FDA approves gardenia (genipin) blue as a natural food color, enhancing options for manufacturers and supporting the shift away from synthetic dyes.
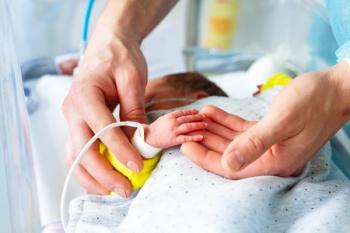
A study reveals declining US neonatal mortality overall, yet highlights rising deaths from fetal malnutrition, emphasizing ongoing disparities in neonatal health outcomes.
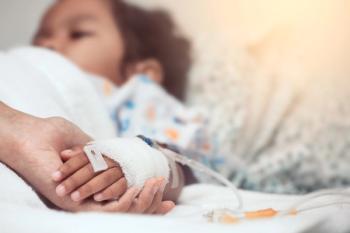
Wearable-derived biorhythms predicted postoperative complications in children up to 3 days early, offering a new tool for remote pediatric monitoring.

The nitric oxide–releasing topical is the first prescription medication approved for at-home treatment of molluscum contagiosum.

A clinical trial found CBD was safe and well-tolerated in autistic boys, with some showing behavioral improvements, though placebo effects were strong.

Cancer-specific summer camps empower children with cancer, fostering community, emotional healing, and safe fun, while providing families much-needed respite and support.

Even low-level lead exposure in early childhood may speed up memory loss, according to a new study using a cognitive task to measure forgetting rates.