
Gluten-free meal plans can be a critical treatment option and necessity for those with celiac disease. But for children with no medical conditions or restrictions? A gluten-free diet should not be the mainstay diet.

Gluten-free meal plans can be a critical treatment option and necessity for those with celiac disease. But for children with no medical conditions or restrictions? A gluten-free diet should not be the mainstay diet.

A high intake of fish and vegetables at 1 year of age was associated with a lower risk of IBD, while consuming sugar-sweetened beverages was linked to a greater risk of developing IBD.

Vivian Hernandez-Truillo, MD, FAAP, FAAAAI, FACAAI; and Theresa Bingemann, MD, provide reaction and commentary regarding recently FDA-approved dupilumab to treat EoE in pediatric patients aged 1 to 11 years.

Deborah Persaud, MD, discusses the background and lead-up to her study examining very early ART in neonates born with HIV-1 and if this treatment could be a step towards ART-free treatment.

Take this quiz and test your knowledge of the American Academy of Pediatrics' recommendations for routine use of influenza vaccines, medications for the prevention and treatment of pediatric influenza.

Based on results from a recent C.S. Mott Poll on Children's Health, parents revealed their own goal-setting helped their children in working toward their respective goals.

A cohort analysis from Japan shows that more visible symptoms—though considered less clinical by experts—may be indicative of heightened suicidal behavior in teenagers.
There are reports that prophylaxis with ibuprofen in the first 12 to 24 hours of life can reduce the risk of severe intraventricular hemorrhage and pulmonary hemorrhage. However, it has not been found to increase survival without neurosensory impairment at 18 months.
A real-world analysis in the United States suggests hospital volume may not be the lone predictor of outcomes in pediatric cardiac surgery.

The approach can improve mental health and various subgroups such as cognitive function, psychological well-being, and more.

Though revisions have been made to the emergency use authorization (EUA), the FDA stated in a press release that the EUA will continue to authorize emergency use in children aged 12 years and older who are at high risk of severe COVID-19.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
A new predictive model from Taiwan suggests cell-based quadrivalent flu vaccines may better egg-based options in reducing disease burden and improving the societal costs of the flu season among children and adolescents.

In this Contemporary Pediatrics Q+A interview, Weily Soong, MD, breaks down FDA-approved tralokinumab-ldrm for patients aged 12 to 17 years with moderate-to-severe atopic dermatitis.

Take this Contemporary Pediatrics quiz, and see if you can correctly diagnose the patient in this case study. Submit your answer to see if you were correct.
Tina Tan, MD, FAAP, FIDSA, FPIDS, tells Contemporary Pediatrics, “This is not new and demonstrates what is known, in that if vaccination rates do not stay at a level that is protective, outbreaks of vaccine preventable diseases will occur.”

The approval makes dupilumab the first and only treatment specifically indicated for eosinophilic esophagitis (EoE) patients aged 1 to 11 years that weigh at least 33 lbs (15 kg).

A pediatric health system discontinues MRSA contact precautions with sustained infection control success, supporting broader consideration while emphasizing the importance of horizontal prevention measures.

In 2021, current-use prevalence of cannabis was lower among male students compared to female students for the first time. This led investigators to conclude that developing interventions that consider protective factors by sex or gender could lead to equity in cannabis reduction strategies among youth.
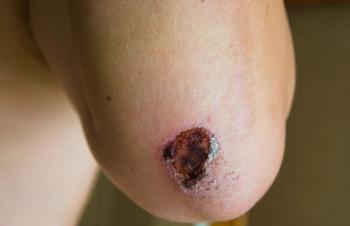
“In pediatric patients, we know that there will be increased scar tissue formation, so it is even more necessary to prevent and reduce the residual burn scars,” Stan Monstrey, former secretary general, president, European Association of Plastic Surgeons, told Contemporary Pediatrics.

A recent study revealed that infants exposed to buprenorphine during the first trimester exhibit a significantly decreased risk of developing congenital malformations associated with opioid exposure compared to infants exposed to methadone.

Decreasing severe adverse safety events (ASEs) could improve the poor outcomes associated with children with out-of-hospital cardiac arrest (OHCA), since 60% had at least 1 ASE.

A new recommendation statement from the Preventive Services Task Force remains inconclusive on the benefits versus risks of asymptomatic screening for speech and language delay disorders.

Considering AI’s potential limitations in diagnosing patients or designing treatment plans, it’s understandable that physicians and other medical professionals would prefer to keep its clinical use at arm’s length for the time being.
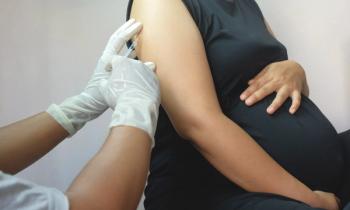
Investigators of a new study published in JAMA Pediatrics concluded that maternal vaccination was safe with regard to neurodevelopment of the child at ages 12 months and 18 months.

A recent cohort study in China reports suggests the structure of green space decreased the relative risk of myopia among school-aged children.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

Though the association of BMI gain and 100% fruit juice in children was “small,” authors concluded their findings support public health guidance to limit consumption of the beverage to prevent overweight and obesity.

An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. Read the full case to see if you can correctly diagnose the patient.

A new study shows that, among the tridemic viruses, RSV was the leading cause of pediatric hospitalizations.