
Manufacturers, hospitals, and clinicians are working quickly to address the shortages of this crucial inhalation solution.

Manufacturers, hospitals, and clinicians are working quickly to address the shortages of this crucial inhalation solution.

Erica Gunderson, PhD, MS, MPH, RD, senior research scientist, Kaiser Permanente Northern California Division of Research, professor of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine discusses how cardiometabolic health and childhood obesity are linked to breastfeeding. Gunderson also discusses receiving the 2022-2023 March of Dimes Agnes Higgins Award at the Pediatric Academic Societies meeting in Washington, DC on April 30, 2023.

In a recent study, offspring were more likely to develop type 1 diabetes if they were born to mothers diagnosed with depression or anxiety during pregnancy.

In a recent study, maternal fever was more common in patients who received epidural analgesia and was associated with adverse neonatal outcomes.

In a recent study, cardiac remodeling and overall function alterations were seen in fetuses with maternal hypothyroidism.
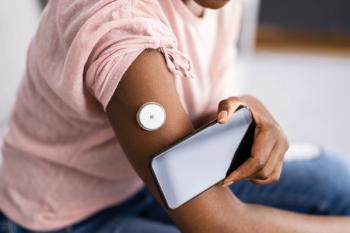
Hemoglobin A1c improved in youths with newly diagnosed type 1 diabetes (T1D) when inclusive continuous glucose monitoring (CGM) was initiated, irrespective of ethnicity or insurance status. According to a study published in Jama Network Open, universal CGM could play a role in reducing disparities for minoritized racial and ethnic groups.

Clinical outcomes of lab values were not statistically different in changes from baseline to 12 months between children with inflammatory bowel disease initiated on the TNF originator or biosimilar.

A recent CDC Morbidity and Mortality Weekly Report revealed concerning results for US high school students' dietary and physical activity behaviors in 2021 compared to 2019.

COVID-19 is no longer a public health emergency of international concern, the World Health Organization (WHO) announced after its 15th International Health Regulations Emergency Meeting.

According to a recently published study, children and adolescents with type 1 diabetes (T1D) and psychiatric disorders have considerable increased odds for educational underachievement compared to those with T1D alone and healthy students.

The study was conducted using 195 randomly selected medical questions posted to Reddit r/AskDocs, an online social media forum where users can post medical questions and verified health care professionals submit answers.

Contemporary Pediatrics® Editorial Advisory Board member Andrew Schuman, MD, clinical assistant professor of pediatrics, Geisel School of Medicine, Dartmouth, discusses whether infant sleep systems are needed for a safe sleeping environment.

Autism prevalence has been an increasing trend even before the COVID-19 pandemic, says J. Thomas Megerian, MD, PhD, FAAP, clinical director, Thompson Autism and Neurodevelopmental Center, Children's Hospital of Orange County. In this Contemporary Pediatrics® interview, watch as he discusses this trend and speaks to the importance of early autism intervention.

In a recent study, centers performing open spina bifida repair saw variations in policies for fetal resuscitation.

In this Contemporary Pediatrics® interview, Jamie Alan, RPh, PharmD, PhD, associate professor, Department of Pharmacology and Toxicology, Michigan State University, talks about the potential impacts red dye No. 3 has on children, and why an assembly bill in California is trying to eliminate it.

According to the study, gay and bisexual adolescents show higher rates of sleep issues and sleep disturbances.

At the 2023 Pediatric Academic Societies meeting, held in Washington, DC, from April 27 to May 1, 2023, the impact that air pollution and climate change has on infants was discussed.

With the recent FDA-approved indication for children aged 2.5 years and older, somapacitan-beco is the first and only once-weekly growth hormone (GH) for children and adults.

Reasons why primary care should incorporate the Rapid Interactive Screen Test for autism spectrum disorder were presented during a session at the 2023 Pediatric Academic Societies Meeting held in Washington, DC.

At the 2023 Pediatric Academic Societies Meeting, presenters provided the benefits of breastfeeding, along with some useful tips for lactation and how to best help your breastfeeding patients.

According to Pfizer, the company’s pneumococcal conjugate vaccine Prevnar 20 has been approved by the FDA to treat infants and children aged 6 weeks to 17 years for the prevention of invasive pneumococcal disease (IPD).

Contemporary Pediatrics® Editorial Advisory Board Member Steven Selbst, MD, gives a glimpse into some research he was involved in regarding physician burnout at the 2023 Pediatric Academic Societies meeting.

Minoryx's leriglitazone showed a decrease in matrix metalloproteinase-9 concentrations in pediatric patients with cerebral adrenoleukodystrophy, according to a 24-week analysis.

Rana Hamdy, MD, discusses being co-chair of a diagnostic stewardship symposium at the 2023 Pediatric Academic Societies meeting, held in Washington, DC from April 27 to May 1.

According to a phase 2 study, remission of macrophage activation syndrome (MAS) secondary to systemic juvenile idiopathic arthritis (sJIA) or adult-onset Still’s disease (AOSD) was achieved in patients taking emapalumab who failed high-dose glucocorticoids.

Contemporary Pediatrics® Editorial Advisory Board Member Steven Selbst, MD, discusses what sessions he is looking forward to the most from this year's Pediatric Academic Societies conference in Washington, DC.

Erica Sood, PhD, Nemours Children’s Health pediatric psychologist, discusses her workshop focused on improving the emotional health of children with chronic conditions, which will be presented at the 2023 Pediatric Academic Societies meeting held in Washington, DC from April 27 to May 1.

In addition, 17 female patients who completed PENFS showed improvements in various symptoms, such as pain, disability, and catastrophizing, while carbohydrate degradation and LCFA synthesis pathways decreased in the post-treatment and follow-up analysis.

Qualitative findings show children with sickle cell disease have the desire to feel normal and utilize personal strengths to manage their condition.

An analysis of 118 infant eyes in India suggests the biosimilar is safe and effective in treating ROP, with about one-third of eyes requiring reinjection at 7 weeks after the first dose.