
Infectious Diseases
Latest News
Latest Videos

CME Content
More News

As families prepare for a new school year, pediatricians are essential in helping parents navigate immunization schedules and school requirements and ensuring vaccine confidence.

As families prepare for a new school year, pediatricians are essential in helping parents navigate immunization schedules and school requirements and ensuring vaccine confidence.
New CDC data show fewer kindergartners are fully vaccinated as exemption rates increased during the 2024-2025 school year.
Pediatricians are urged to follow 2024 guidelines amid ACIP upheaval, vaccine hesitancy, and access issues as children head back to school.
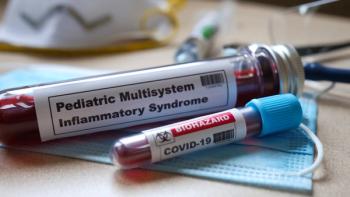
“While our study is small, its results are powerful and have implications not only for MIS-C, but potentially for long COVID,” said lead author Lael Yonker, MD.
Of the 253 influenza-related pediatric deaths through June 21, 2025, 42.7% occurred in children without a high-risk medical condition.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Lori Handy, MD, MSCE, highlights how pediatricians can better prepare families and systems for missed vaccines during the back-to-school rush.

This week, HHS Secretary Robert F. Kennedy Jr signed the ACIP's recommendation to remove the mercury-based preservative from all influenza vaccines.
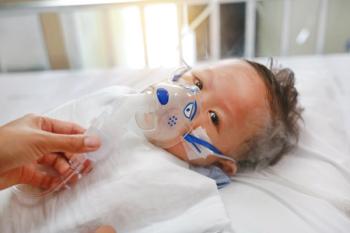
Nirsevimab reduced RSV-related illness by up to 98% and lowered the amount of hospitalizations and health care use among healthy term infants in a real-world study.

In this episode of Infectious Insights, our host Tina Tan, MD, is joined by general pediatrician Candice Jones, MD, to discuss an unprecedented vaccine season.

Lori Handy, MD, MSCE, emphasizes the role of school entry as a critical time for ensuring children are caught up on routine vaccinations.

Discover the latest in pediatric infectious diseases with Infectious Insights, a new podcast series hosted by expert Tina Tan, MD, FIDSA, FPIDS, FAAP.

Early antibiotic exposure disrupts infant immune development by altering gut microbiota, highlighting inosine's potential as a therapeutic target for immune restoration.
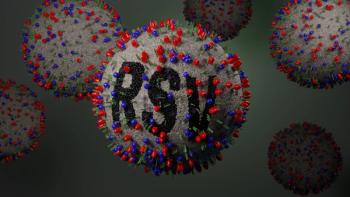
A newly-published study identified specific groups of higher-risk children that could benefit most from monoclonal antibodies.
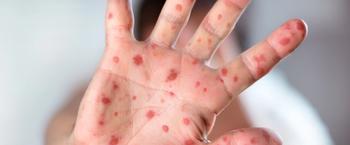
A week into July 2025, the total reported cases in the United States have surpassed the peak of 1274 cases recorded for all 2019.

Six medical societies, including the American Academy of Pediatrics, are suing the HHS and its Secretary to "defend vaccine policy."

Mycoplasma pneumoniae caused 1 in 2 pediatric pneumonia hospitalizations in 2024, with sharp increases seen across all age groups, according to a recent CDC MMWR report.

The final recommendation sign off decision will go to HHS secretary Robert F. Kennedy Jr.
The American Academy of Pediatrics will continue to hold its own childhood vaccine schedule, as it has since the 1930s.

Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, shares her thoughts on how PNPs can implement evidence-based strategies to encourage parents to vaccinate their children.

Octavio Ramilo, MD, joins us once again to discuss potential benefits of RSV vaccination beyond disease prevention and to explain how to speak with patients about vaccines and uptake.

In this video, the last in a 3-part series, panelists discuss future directions in the field and the importance of multispecialty collaboration.

In this video, part 2 in a 3-part series, panelists discuss clesrovimab's recent approval and gaps in clinician education.

In this video, the first in a 3-part series, panelists discuss recent advancements in RSV management.


















