
In this interview, Suzanne Hollander, MS, RD, LDN, discusses the importance of collaborative care in PKU and the impact of a new potential treatment.

In this interview, Suzanne Hollander, MS, RD, LDN, discusses the importance of collaborative care in PKU and the impact of a new potential treatment.
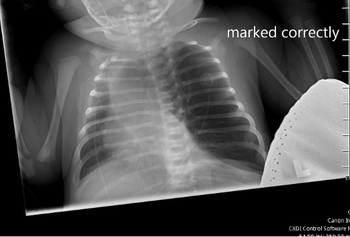
On physical examination, the patient appeared to be in significant respiratory distress. Her oxygen saturation was 96% on high-flow nasal cannula.

Prescriptions for the epinephrine nasal spray are now available for patients aged 4 years and up who weigh 33 to < 66 lbs.

A review of the clinical potential of PTC Therapeutics' sepiapterin as a novel treatment for phenylketonuria (PKU).

Even before the change in national leadership, access to adolescent gender-affirming care was becoming more difficult, with many barriers faced by these at-risk patients.
Current prevention strategies include 2 injectables for infants and young children, namely palivizumab and nirsevimab.

Research shows that nearly 80% of foster children have at least 1 significant mental health need.

Joshua Feder, MD, highlights the need for psychiatry to better integrate sensory processing research into care strategies beyond autism.
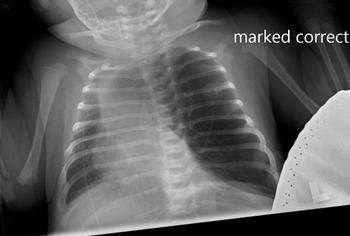
Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Watch some of our top clips from a number of discussions with experts who presented at PAS 2025.

Mona Shahriari, MD, discusses how RAD 2025 can equip providers with tools to improve atopic dermatitis outcomes.

Colleen Sloan, PA-C, RDN, shares the importance of breakfast and the reasoning to why kids skip this essential meal.
Nirsevimab significantly reduces RSV-related hospitalizations, ICU admissions, and LRTIs in infants, but doesn’t shorten hospital stays, according to a recent study.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from April 2025.

In this article, we recap our top stories, expert interviews, and Q+A discussions from the 2025 Pediatric Academic Societies meeting.

Lea Widdice, MD, details her recent presentation at PAS 2025 on OTC STI testing within pediatrics.

Stealth BioTherapeutics' CEO stated, "We hope to gain more information on the revised action date in the coming days."

The human FcRn-blocking monoclonal antibody is indicated for gMG patients aged 12 years and older.

A PAS 2025 study by Mazar and Westmoreland shows Zika virus can eliminate both local and distant tumors in neuroblastoma mouse models.

A UK study links maternal anemia in early pregnancy with increased congenital heart disease risk in offspring.

The decision makes pz-cel the first and only autologous cell-based gene therapy for the treatment of wounds in adult and pediatric patients with RDEB.

New research led by Geetika Kennady, MD, FAAP, showed that 10.6% of newborns have iron deficiency, supporting a push for earlier screening.

"We've updated our internal clinical pathways to recommend 5 days for kids hospitalized with uncomplicated pneumonia," Cotter noted.

At PAS 2025, Jesse Hinckley, MD, PhD, presented data showing cannabis use sharply increases odds of depression and suicidal behavior in youth.

New model refines prevalence estimates, reduces misdiagnoses compared to the current coding system.

A recent study found reduced preterm birth, complications, and NICU use in large Tennessee Medicaid cohort.

Jennifer Nestor, MD, evaluated a digital stethoscope to validate pediatric breath sound recordings for potential machine learning algorithm development.

Study finds education from multiple sources increases parent inquiry about firearms.

New data from national survey highlights disparities and need for focused prevention strategies.

In addition, data presented at PAS revealed a predictable pharmacokinetic profile in the newborn population, consistent with adult humans.