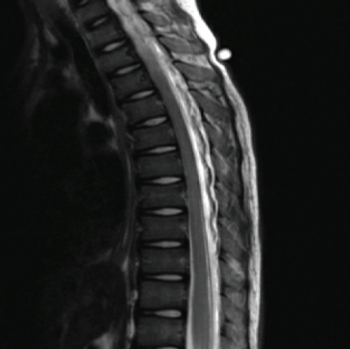
An 11-year-old boy with a history of asthma and allergic rhinitis presented to the emergency department (ED) with worsening fatigue, minimal responsivity to external stimuli, and diffuse muscle weakness for 2 months.

An 11-year-old boy with a history of asthma and allergic rhinitis presented to the emergency department (ED) with worsening fatigue, minimal responsivity to external stimuli, and diffuse muscle weakness for 2 months.

Suicide was among the top 9 leading causes of death for people aged 10 to 64 years, and was the second leading cause of death for individuals aged 10 to 14 years and 25 to 34 years in 2022.

Current treatment is primarily supportive care consisting of increased fluid intake to dilute oxalate in the urine, along with pyridoxine, or vitamin B6, to reduce oxalate production.

Jon Matthew Farber, MD, offers quick thoughts on a recent study regarding bone health and activity among adolescents withJIA.

Wondering how to counsel parents on keeping their child’s immune system healthy through nutrition? Check out these clinical pearls from Colleen Sloan, PPA-C, RDN.

The formula company issued a recall of the products on May 24, 2024, though they were not in compliance with all of the FDA’s infant formula regulations.
A pair of medical directors involved in the transplant clinical space provide commentary on heart and lung transplants in the pediatric population.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

Using school-based approaches and staying in close contact with school personnel can increase overall care for the child.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the August 2024 issue of Contemporary Pediatrics.

The most common comorbid conditions were endocrinopathies (10%), trisomy 21(5%), and attention-deficit/hyperactivity disorder (4%).
Nine of 32 (28.1%) patients in the mitapivat arm achieved a transfusion reduction response compared to 11.8% of patients in the placebo arm.

Diamyd to improve glycemic control in recently diagnosed stage 3 Type 1 Diabetes patients with the genotype HLA DR3-DQ2 was granted Fast Track designation by the FDA in February 2024.

Erica Prochaska, MD, highlights a recent study that aimed to estimate the rate of HOB among infants admitted to the NICU, measure the association of HOB risk with birth weight group and postnatal age, and estimate HOB-attributable mortality.

These data highlighted new findings on the experiences of children aged 6 to 11 with uncontrolled type 2 asthma treated with dupilumab.

With the expanded label, Palforzia is now approved to treat individuals aged 1 to 17 years with a confirmed peanut allergy diagnosis, after the treatment was originally approved in 4-to-17-year-olds in January 2020.
A recent study reveals a direct association between higher maternal body mass index and the risk of sudden unexpected infant death, underscoring the need for further research into the causal mechanisms.

A secondary analysis of a randomized trial points to limited benefits on the microbiota.

"Taken together, our findings suggest the relative protection associated with having a mother versus father with type 1 diabetes is a long-term effect that extends into adult life," stated study investigators.

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.
Overall, mortality rates among youth in the United States were higher than 16 comparison countries used in the study.

ConSynance Therapeutics' CSTI-500, an innovative Triple Monoamine Reuptake Inhibitor, has received FDA's Rare Pediatric Disease Designation for treating Prader-Willi Syndrome in children.

From baseline to final assessment, all 7 matched cerliponase alfa-treated children under 3 years of age maintained a motor score of 3, representing a "grossly normal gait, signifying a delay in disease onset," stated BioMarin.

Initial approval for maralixibat was granted on March 13, 2024, to treat PFIC patients aged 5 years and older.

These data highlighted the association of moderate air pollution exposure during pregnancy, infancy, and early childhood on pulmonary function at school age.

The third and final episode in our series looks at what is in the pipeline as well as a discussion around FDA guidance.

Merck announced clesrovimab met all primary safety and efficacy endpoints, with additional detailed findings to be presented at an upcoming scientific congress.

With the acceptance of the BLA, a new PDUFA date of January 7, 2025 has been set.
Risk for cataract development was assessed at specific follow-up durations of 1 year, 2 years, 5 years, and 20 years following the index date, with an increased risk of cataracts development among patients aged younger than 18 years present at each time point.

The investigative team noted that teenagers with emotional and social support are better off to handle stressors such as biological and social transition, and are less likely to experience a variety of adverse physical and mental health outcomes.