
Lawrence Eichenfield, MD, highlights the sNDA submission of tapinarof cream, 1% for the topical treatment of atopic dermatitis in children aged 2 years and older.

Joshua Fitch is the senior editor for Contemporary Pediatrics. He joined the brand in March of 2023 as an editor before being promoted to senior editor in January 2024. Fitch graduated from Youngstown State University in Youngstown, Ohio in 2020 with a degree in telecommunications and journalism. He started his career as a news and sports videographer before becoming an on-air sports anchor at the NBC-affiliated news station in Youngstown. Fitch briefly worked as a national content writer for a Chicago-based national television station before joining the Contemporary Pediatrics team. He can be reached at: jfitch@mjhlifesciences.com.

Lawrence Eichenfield, MD, highlights the sNDA submission of tapinarof cream, 1% for the topical treatment of atopic dermatitis in children aged 2 years and older.

Promoting mental health and implementing stress-reducing scenarios for adolescents could lessen motivating factors for substance use.

Results demonstrated that foster care involvement does vary based on age and sex, with Black youths and females disproportionately effected.

The sNDA submission follows additional positive topline data that was presented in Janurary 2024, highlighting an open-label, long-term extension study evaluating tapinarof cream, 1%.

Compared to children with no maternal tobacco usage during pregnancy, those exposed had associations to childhood neurocognition deficits.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

The twice-daily oral corticosteroid is indicated for 12 weeks of treatment.

Joshua Feder, MD, explains how developmental care models for autism care can benefit children with autism, providers, parents, and other family members.

Samantha Olson, MPH, explains the important role of a strong recommendation for maternal influenza vaccination.

Deborah Persaud, MD, details results from her study that aimed to reduce HIV reservoirs in neonates with very early antiretroviral therapy.

Read the case and take your best guess at diagnosing this 4-year-old patient.

Samantha Olson, MPH, breaks down a study highlighting the effectiveness of maternal influenza vaccination and its respective association with influenza-related hospitalizations in infants.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

Nirsevimab was approved by the FDA on July 17, 2023, ahead of the traditional RSV season, though in October, the Centers for Disease Control and Prevention (CDC) recommended it be prioritized for the highest-risk infants amid limited availability.

Gluten-free meal plans can be a critical treatment option and necessity for those with celiac disease. But for children with no medical conditions or restrictions? A gluten-free diet should not be the mainstay diet.

Vivian Hernandez-Truillo, MD, FAAP, FAAAAI, FACAAI; and Theresa Bingemann, MD, provide reaction and commentary regarding recently FDA-approved dupilumab to treat EoE in pediatric patients aged 1 to 11 years.

Deborah Persaud, MD, discusses the background and lead-up to her study examining very early ART in neonates born with HIV-1 and if this treatment could be a step towards ART-free treatment.

Take this quiz and test your knowledge of the American Academy of Pediatrics' recommendations for routine use of influenza vaccines, medications for the prevention and treatment of pediatric influenza.

Based on results from a recent C.S. Mott Poll on Children's Health, parents revealed their own goal-setting helped their children in working toward their respective goals.
There are reports that prophylaxis with ibuprofen in the first 12 to 24 hours of life can reduce the risk of severe intraventricular hemorrhage and pulmonary hemorrhage. However, it has not been found to increase survival without neurosensory impairment at 18 months.

The approach can improve mental health and various subgroups such as cognitive function, psychological well-being, and more.

Though revisions have been made to the emergency use authorization (EUA), the FDA stated in a press release that the EUA will continue to authorize emergency use in children aged 12 years and older who are at high risk of severe COVID-19.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

In this Contemporary Pediatrics Q+A interview, Weily Soong, MD, breaks down FDA-approved tralokinumab-ldrm for patients aged 12 to 17 years with moderate-to-severe atopic dermatitis.

Take this Contemporary Pediatrics quiz, and see if you can correctly diagnose the patient in this case study. Submit your answer to see if you were correct.
Tina Tan, MD, FAAP, FIDSA, FPIDS, tells Contemporary Pediatrics, “This is not new and demonstrates what is known, in that if vaccination rates do not stay at a level that is protective, outbreaks of vaccine preventable diseases will occur.”

The approval makes dupilumab the first and only treatment specifically indicated for eosinophilic esophagitis (EoE) patients aged 1 to 11 years that weigh at least 33 lbs (15 kg).

In 2021, current-use prevalence of cannabis was lower among male students compared to female students for the first time. This led investigators to conclude that developing interventions that consider protective factors by sex or gender could lead to equity in cannabis reduction strategies among youth.
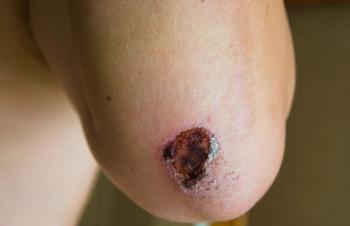
“In pediatric patients, we know that there will be increased scar tissue formation, so it is even more necessary to prevent and reduce the residual burn scars,” Stan Monstrey, former secretary general, president, European Association of Plastic Surgeons, told Contemporary Pediatrics.

Decreasing severe adverse safety events (ASEs) could improve the poor outcomes associated with children with out-of-hospital cardiac arrest (OHCA), since 60% had at least 1 ASE.