Phase 3 results show oral zoliflodacin was non-inferior to ceftriaxone and azithromycin for uncomplicated urogenital gonorrhea.

Phase 3 results show oral zoliflodacin was non-inferior to ceftriaxone and azithromycin for uncomplicated urogenital gonorrhea.

This approval of gepotidacin is the first in a new antibiotic class for the treatment of gonorrhoea approved in over 3 decades.

According to multiple reports, the FDA is investigating the safety of a pair of already-approved protective treatments for respiratory syncytial virus (RSV).

See the full case of our most recent puzzler quiz, and view the correct diagnosis of this slender teen with a growing abdomen and shrinking height.

Following an updated policy from the American Academy of Child and Adolescent Psychiatry, Joshua Feder, MD, explains why providers should present DRBIs and NDBIs as options for autism care.

Waskyra becomes the first FDA-approved gene therapy for Wiskott-Aldrich syndrome, offering hope to patients with this rare immunodeficiency.
“We took a different approach by looking at colonizing bacteria, and we found vaccination reduced antimicrobial resistance through a completely different mechanism," said Brooke Ramay, lead author of the study.
A comprehensive Pediatrics review reports no credible evidence linking aluminum-adjuvanted vaccines with autism, neurotoxicity, allergy, or autoimmune disease.

Researchers report that cheek swabs can capture early microscopic changes of ACM, offering a noninvasive path to earlier diagnosis and intervention.

The FDA approved omidubicel on December 8, 2025, the same day parent company Ayrmid announced positive results at the 2025 American Society of Hematology Annual Meeting and Exposition.
Marstacimab’s once-weekly subcutaneous administration demonstrated clinically meaningful superiority in key bleeding outcomes for adolescents and adults with hemophilia A or B.


Christine Eng, MD, discusses how earlier genome testing, multi-omic tools, rapid sequencing, and improved access can shorten diagnostic timelines and guide care.
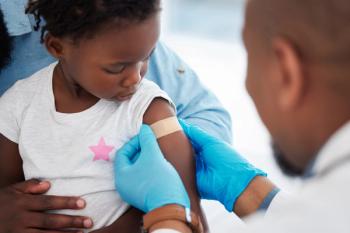
State repeals of nonmedical vaccine exemptions were linked to higher kindergarten vaccination rates, with minimal substitution toward medical exemptions.


The ACIP committee voted to pass both resolutions to update its recommendation on the hepatitis B vaccine for infants.

Christine Eng, MD, discusses insurance, access, logistics, and result interpretation challenges affecting effective use of exome and genome sequencing.

"For those not receiving the HBV birth dose, it is suggested that the initial dose is administered no earlier than 2 months of age," reads part of vote 1.

FDA approves expanded indication for MED-EL cochlear implants, allowing use in infants 7 months or older with bilateral SNHL and broadening pediatric eligibility.

New AAP guidance supports early use of exome and genome sequencing, offering higher diagnostic yield and faster results for children with developmental delay and ID.

Higher maternal vitamin D levels in pregnancy were associated with reduced odds of early childhood caries and lower dmft scores in offspring.

Joshua Feder, MD, highlights how interest-based, blended, and ABA strategies can all be effective options when working with a child with autism.

New US modeling study results find nirsevimab would avert the most infant RSV-LRTD events and hospitalizations compared with clesrovimab or RSVpreF maternal vaccination.

Joshua Feder, MD, explains how AACAP’s revised policy aims to improve access, insurance coverage, and family-centered decision-making for autism care.

Alice Hoyt, MD, FAAAI, underscored three core recommendations for improving pediatric food allergy management, emphasizing coordinated care and reliable access to epinephrine.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from November 2025.

Jonathan Flyer, MD, emphasizes that early detection of FH through guideline-recommended universal screening can be lifesaving and supports broader family identification.

Take a quick look at everything you may have missed this month, including the top FDA approvals and latest clinical updates.

“It represents the first time an in vivo HSC-directed gene insertion therapy has been evaluated in a patient," said CEO Jim Burns in a statement.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.