
A new clinical trial has found that omalizumab (Xolair; Genetech, Novartis) is more effective than oral immunotherapy (OIT) in treating multi-food allergy in individuals with severe allergic reactions to small amounts of common food allergens.

A new clinical trial has found that omalizumab (Xolair; Genetech, Novartis) is more effective than oral immunotherapy (OIT) in treating multi-food allergy in individuals with severe allergic reactions to small amounts of common food allergens.

Diazoxide choline has been granted breakthrough, fast track, and orphan drug designations in the United States.

13-year-old female with altered mental status, headache and emesis. What is the diagnosis?

In this Q+A interview, Craig McDonald, MD, discusses the interim, 90-day data from the phase 1/2 INSPIRE DUCHENNE trial, evaluating SGT-003, a gene therapy candidate for DMD.

The approval marks a vital step toward care for urinary tract infections, helping to reduce recurrence and improve patients’ quality of life.

Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Discover mental health screening tools for the pediatric primary care provider.
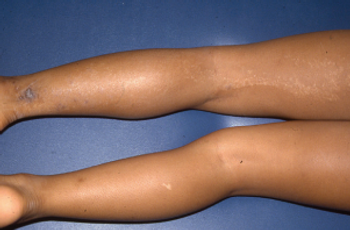
What's the diagnosis of this linear eruption on an adolescent girl's leg?

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
Pediatric cancer survivors can be vulnerable to cardiovascular disease in the short- or long-term with increased recognition of cardiotoxic cancer treatments, as 5-year survival rates for children are greater than 85%.

With 1 in 7 adolescents worldwide diagnosed with mental health disorders, emerging research suggests that diet is a powerful yet often overlooked therapeutic tool.

Walker and Harris emphasize the importance of early hearing loss detection, screening, and intervention to support optimal child development.

The PANDAS/PANS questionnaire is based on symptoms and comorbidities described in the literature and is a self-report completed by parents of children younger than 18.

Take your best guess and try to diagnose this 9-year-old girl's linear eruption on the back of her left thigh and leg. Stay tuned for the full case presentation and correct diagnosis!

Freely available measures may seem initially like a bargain, but their excess demands on staff time not only are expensive but often deter one of the central goals of well-child visits.

Peter S. Jensen, MD, discusses the risks and benefits of social media use by children, emphasizing the need for parental oversight and national guidelines.

Results were from the phase 3 STEER study among a broad population of patients with SMA aged 2 to under 18 years.

The federal agencies are encouraging companies to develop new infant formulas and clarify opportunities to inform consumers about formula ingredients.

Danielle Van Damme, DNP, CPNP-AC, discussed key updates from the 2024 IDSA guideline on diagnosing complicated intra-abdominal infections, emphasizing ultrasound as the preferred imaging modality.

Prior to approval on February 14, 2025, the only FDA approved vaccine for chikungunya protection was indicated for 18 years and up.

"Bottom line, previous surgery for endometriosis is not a contraindication to the use of an IUD (usually with HT) if the patient and you agree that this is the best option," said Jon Matthew Farber, MD.

Karen Y. Capusan, DNP, CPNP-PC, explored RSV’s shifting epidemiology, seasonal trends, and new vaccines’ role in reducing hospitalizations at the 2025 NAPNAP National Conference.

Pediatricians face rising demands in specialty care and mental health, with programs such as REACH helping them manage these challenges.

At the 2025 NAPNAP Conference in Chicago, Jessica Spruit, DNP, CPNP-AC, FAANP, discussed the AAP's first clinical practice guideline on opioid prescribing.

Bobbie Monaco, MSN, CPNP-PC, provided a recap of her NAPNAP session regarding period poverty, and called for continued advocacy and awareness in the United States and globally.

Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, comments on the ongoing measles outbreaks and encourages advocation for vaccines and accurate information.

Mary Koslap-Petraco, DNP, PPCNP-BC, CPNP, FAANP, highlighted key updates on influenza, COVID-19, and RSV vaccines at the 2025 NAPNAP National Conference.
A nurse-led intervention increased HPV vaccination rates among Hispanic adolescents in a rural North Carolina clinic, according to research presented at the 2025 NAPNAP conference.

Early anxiety screening in pediatric primary care improves detection, outcomes, and access to mental health support starting at age 8.

Tina Tan, MD, FIDSA, FPIDS, FAAP, comments on the FDA's recent selection of the 2025-2026 influenza vaccine compositions, without the CDC's ACIP meeting and FDA's VRBPAC meeting.