
News


The agency approved a monthly dosing option for select patients acorss all approved indications, including wAMD, DME, DR, and RVO.

Alice Hoyt, MD, FAAAI, discussed the Early Childhood Anaphylaxis Collaborative’s efforts to improve anaphylaxis preparedness in early childcare settings.

Genetic testing in at-risk children can improve monitoring, guide activity choices, reduce stress, and prepare families for future therapies.

"What is the possibility for your individual child that you're taking care of as a patient, as a family member advocate?" said Oliva Kim-McManus, MD.

“This label expansion helps close a long-standing gap in the treatment of pediatric patients with hereditary antithrombin deficiency,” said George M. Rodgers, III, MD, PhD.

In this interview from the 2025 Child Neurology Society meeting, Olivia Kim-McManus explains how far the gene therapy and genetic testing space has come related to pediatric neurology.

The target action date for roflumilast cream 0.3% for plaque psoriasis in children 2 to 5 years is set for June 29, 2026.

Long-term data show sustained survival, immune recovery, and metabolic correction after lentiviral gene therapy for ADA-SCID, with no vector-related safety events.

National CPS data from 2012-2023 show declines in confirmed child maltreatment, but disparities remained, particularly among Black and female children.

FDA adds boxed warning to Elevidys and limits use to ambulatory DMD patients ≥4 years after reports of fatal acute liver failure in non-ambulatory patients.
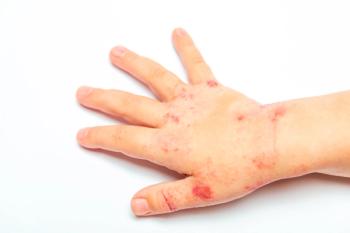
Sub-analysis of ADORING trials shows VTAMA cream improved skin clearance, symptoms, and patient-reported outcomes in children aged 2-17.

A review found no link between prenatal acetaminophen exposure and offspring neurodevelopmental disorders.

Valeria C. Cohran, MD, emphasized the importance of close collaboration between intestinal rehab programs and general pediatricians to optimize care for children with intestinal failure.

Older age, longer remaining intestine, and presence of an ileocecal valve were linked with a greater likelihood of achieving sustained enteral autonomy in children with short bowel syndrome-associated intestinal failure.

Discover the critical role of physical exams in diagnosing rare conditions like spontaneous pneumomediastinum through unique clinical signs.

Study of 99 pediatric patients found clinically significant CNVs in 30% of cases, supporting CMA as a first-tier test for developmental delay and ID.

Dupilumab maintained histologic and endoscopic improvements across all pediatric age groups with EoE, according to Joshua Wechsler, MD.

New research suggests shorter antimicrobial treatments for pediatric urinary tract infections may be effective, challenging traditional 10-day courses.

Can you guess the diagnosis?

Test your knowledge of updated AAP recommendations for genetic evaluation of children with developmental delay and intellectual disability.

A survey reveals pediatric providers' limited changes in emergency contraception practices post-Dobbs, highlighting knowledge gaps and barriers to prescribing.

Fifteen infants with suspected or confirmed infant botulism and a confirmed exposure to Byheart Whole Nutrition infant formula have been reported from 12 states.

The patient was discharged after symptoms improved with antiemetics and pain medications.

FDA grants Breakthrough Device Designation to GeneDx genome and exome tests supporting diagnosis of life-threatening genetic disorders.

In this Contemporary Pediatrics video interview, Dale Lee, MD, highlights how pediatricians can be involved in celiac disease follow-up care.

Thomas Wallach, MD, discusses phase 3 safety data of tenapanor in pediatric patients with IBS-C, presented at the 2025 NASPGHAN Annual Meeting.

Long-term analysis of the PEDFIC studies presented at NASPGHAN 2025 demonstrated sustained reductions in bile acids and pruritus with odevixibat in FIC1 deficiency.

Julie Khlevner, MD, AGAF, explains the impact of the FDA approval of linaclotide for pediatric IBS-C
Julie Khlevner, MD, AGAF, highlights that FDA approval of linaclotide provides the first pharmacologic therapy for pediatric IBS-C.

At NASPGHAN 2025, investigators presented 52-week safety data from an ongoing phase 3 trial of linaclotide, the first approved treatment for patients aged 7 and older with IBS-C.

